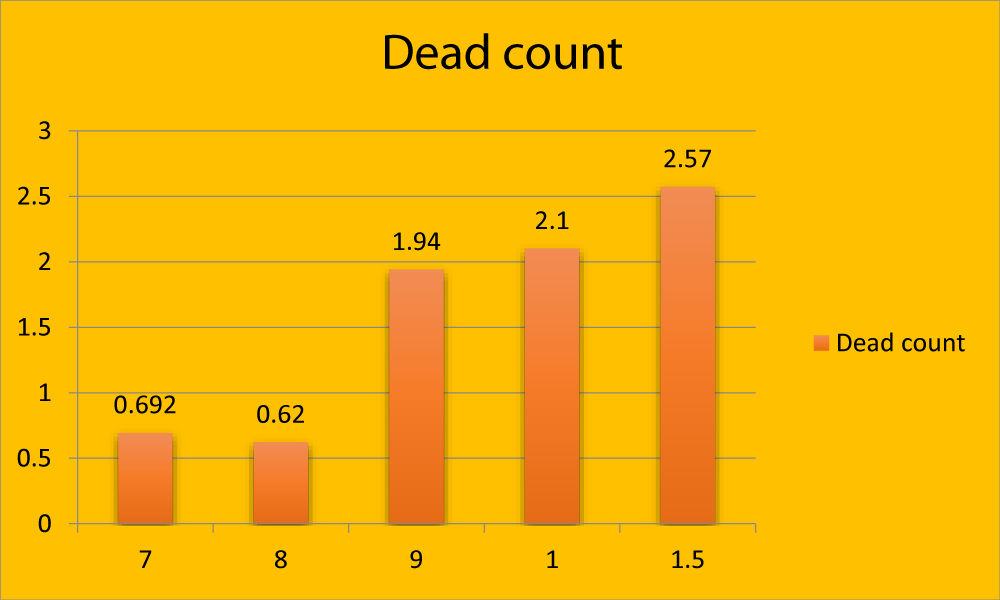More Information
Submitted: January 07, 2022 | Approved: March 31, 2022 | Published: April 01, 2022
How to cite this article: Nissar S, Ramesh G, Basha SH, Kannan TA, Hussain S. Age-related changes in cell yield and viability of feline Adipose Tissue-Derived Mesenchymal Stem (fAD-MSCs). J Stem Cell Ther Transplant. 2022; 6: 001-004.
DOI: 10.29328/journal.jsctt.1001024
Copyright License: © 2022 Nissar S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Adipose tissue; Cell viability; Cell yield; Feline; Mesenchymal; Omental; Stem cell; Regenerative medicine
Age-related changes in cell yield and viability of feline Adipose Tissue-Derived Mesenchymal Stem (fAD-MSCs)
Shazia Nissar1*, Geetha Ramesh2, Sabiha Hayath Basha3, TA Kannan4 and Shahid Hussain5
1Ph. D. Scholar, Department of Veterinary Anatomy, Madras Veterinary College, Chennai, India
2Professor & Head, Department of Veterinary Anatomy, Madras Veterinary College, Chennai, India
3Professor and Head, Department of Veterinary Anatomy, Veterinary College and Research Institute Salem, India
4Professor and Head, Education Cell, Madras Veterinary College, Chennai, India
5Assistant Professor, Division of Veterinary Clinical Complex, SKUAST, Kashmir, India
*Address for Correspondence: Shazia Nissar, Ph.D. Scholar, Department of Veterinary Anatomy, Madras Veterinary College, Chennai, India, Email: [email protected]
In the present study, omental adipose tissue was collected from, the animals that underwent ovariectomy and ovariohysterectomy, surgical procedures, at the age of seven months to 11/2 years of age groups. The sample was digested with 0.1% (W/V) collagenase type I and transferred to a beaker with a magnetic stirrer and kept in a stirrer with 600 rpm at 37 °C for 30 minutes. The viability of the cell was evaluated by the trypan blue exclusion method using a hemocytometer. Trypan blue had a high affinity to nuclear DNA, which traverse the member in a dead cell and dye it blue. In the present study, the cell yield of fAD-MSCs was 8.15 ± 0.68, 6.55 ± 0.26, 4.85 ± 0.42, 3.90 ± 0.34, and 3.51 ± 0.43 in different age groups viz., 7,8,9 month 1 and 1½ year respectively. In younger age groups, cell yield and viability percentage were more than in animals of higher age groups. In the younger age group, stem cells proliferation status is considered potent for therapeutic application.
The potential impact of the field of regenerative medicine has a multitude of applications through stem cell biology. The expansion of our basic knowledge of stem cell biology will greatly impact scientific areas including development and aging as well as provide a foundation for the development of tissue engineering (Merok and Sherley, 2001).
Stem cell biology is one of the most active areas in biomedical research today. Stem cells are unspecialized cells that have the ability to self-renew and give rise to at least one more committed progenitor or differentiated cell type.
Stem cells can be classified according to their differentiation potential as totipotent, pluripotent, multipotent, and unipotent; this differentiation potential or stemness is likely to exist as a gradient, in the organism or tissue in which they reside.
In mammals, only the zygote and cells of the cleavage-stage embryo are totipotent (up to the four-eight cell-stage) and can generate an entire organism, including extraembryonic tissues.
Adipose tissue, as a stem cell source, is ubiquitously available and has several advantages compared to other sources. It is easily accessible in large quantities with minimal invasive harvesting procedure and the isolation of these adipose-derived mesenchymal stem cells (AD-MSCs) yields a high amount of stem cells, which is essential for stem-cell-based therapies and tissue engineering. AD-MSCs are indeed multipotent somatic stem cells exhibiting growth kinetics, plasticity and proved to induce efficient tissue regeneration in several biomedical applications. Cultures of AT-MSCs were easier to generate because of their higher intrinsic proliferative rate and maintain their phenotypes. Hence, it is used for tissue engineering and regenerative medical applications (Bunnell et al. 2008).
Collection of feline adipose tissue
Omental adipose tissue samples were collected from female cats of different age groups during ovariectomy or ovariohysterectomy, which is a sample collection bottle that contained phosphate buffer saline (PBS). It was processed within 30 minutes of the collection of the sample. Adipose tissue was weighted by using an electronic weighing balance and washed three to four times with an equal volume of normal saline.
Isolation of feline adipose-derived mesenchymal stem cells (fAD-MSCs).
Enzymatic digestion of the adipose tissue
- The weighted adipose tissue was rinsed with phosphate-buffered saline, to remove blood, small vessels, and connective tissue. Cleaned adipose tissue was cut into small pieces by using sterile forceps and a surgical blade.
- Samples were digested with 0.1 percent (W/V) collagenase type I (SIGMA) and transferred to a beaker with a magnetic stirrer and kept in a stirrer with 600 rpm at 37 oC for 30 minutes (Kono, et al. 2014).
- After digestion stromal vascular fraction (SVF) was pipetted out and neutralized with an equal volume of Dulbecco’s modified Eagle medium-high glucose (DMEM-HG) with fetal bovine serum (FBS) and antibiotic solution (45 ml of DMEM-HG, 5ML of 10 percent FBS, 0.5ml of antibiotic solution and non-essential amino acids was added to it [1].
- Finally, it was centrifuged at 2500 rpm for 10 minutes, the supernatant was discarded and the pellet was resuspended in 1ml of culture media (DMEM-HG with 10 percent FBS, 0.5 ml of antibiotic solution, and non-essential amino acids) to get cell suspension [2].
Viability of the cell
Cell viability and total cell density were determined by a 0.4% Trypan blue exclusion test. 10µl of cell suspension was added to 10 µl of Trypan blue and from that mixture, 10 µl was taken to check the viability of cells [3]. The cells were counted manually in Neubauer’s hemocytometer. The total cell density for seeding was calculated using the formula:
(Total cell number × dilution factor)/104
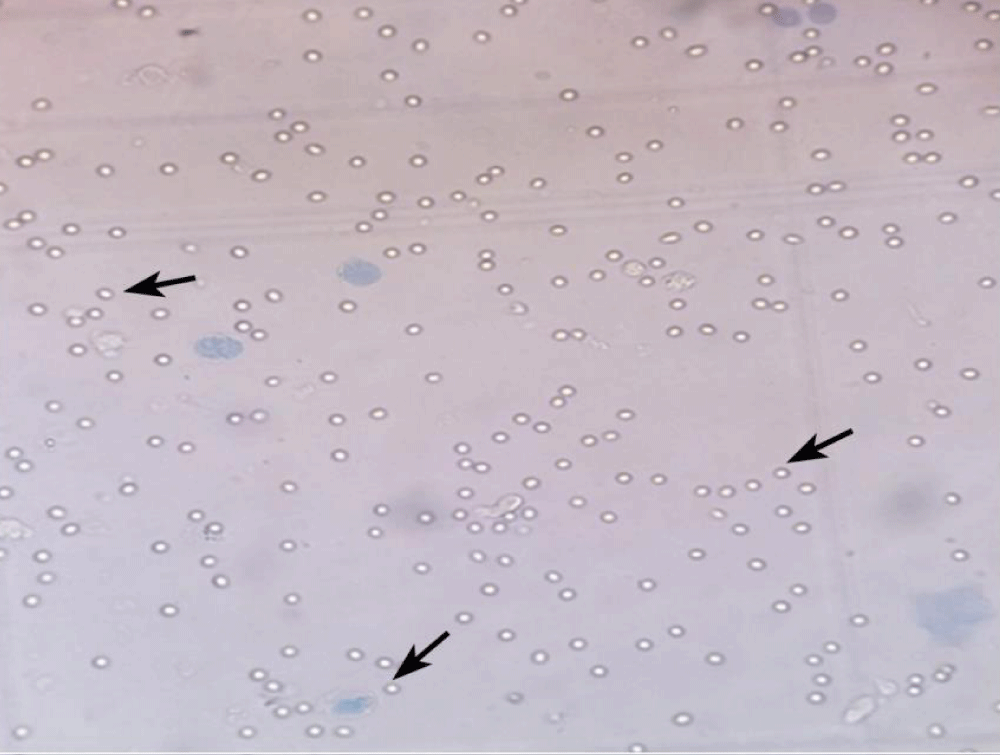
In the present study, feline adipose-derived mesenchymal stem cells (fAD-MSCs) were processed and subjected to collagenase type I (0.1% W/V) treatment for isolation of stromal vascular fraction (SVF). In the present study, cell viability was confirmed by Trypan blue exclusion test (Plate 1).
Plate 1: Photograph showing viable fAD-MSCs in omental fat. Trypan blue exclusion test X 100.
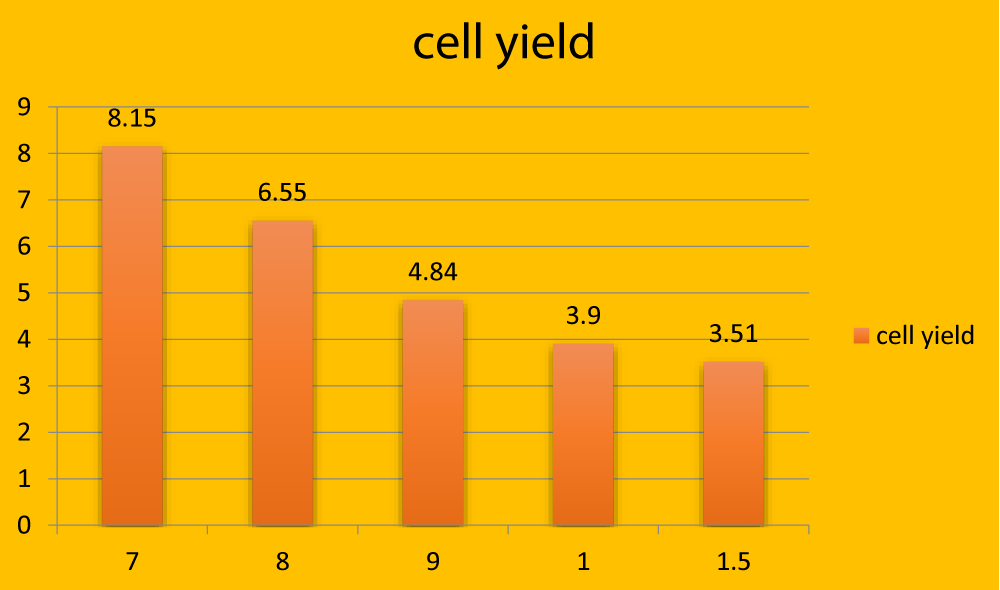
The live-cell were observed with transparent cytoplasm and intact nucleus. The cytoplasm of dead cells was stained with trypan blue. The cell yield was calculated by counting viable cells using Neubauer’s chambers. Cell yield was higher in young age groups (Table 1 and Figures 1-4). In the present study, the size of CFUs (Colony Forming Unit) and the number of cells in each CFUs were found to be increased as culture advanced in all the age groups The cell yield of omental adipose tissue was 8.15 ± 0.68, 6.55 ± 0.26, 4.85 ± 0.42, 3.90 ± 0.34 and 3.51 ± 0.43 in different age groups 7, 8, 9 months 1 and 1½ year respectively. This indicated that in younger age groups, the proliferation rate was found to be more when compared to an adult. Based on the result of the present study, fAD-MSCs isolated from seven months to 11/2 years of age might be anticipated to be a more useful cell source for tissue engineering and regenerative medicine application.
| Table 1: Cell yield from fAD-MSCs in different age groups. | ||||
| Age | Cell yield (x106) (Mean ± SE) |
Viable cell count (x106) (Mean ± SE) |
Dead cell count (x106) (Mean ± SE) |
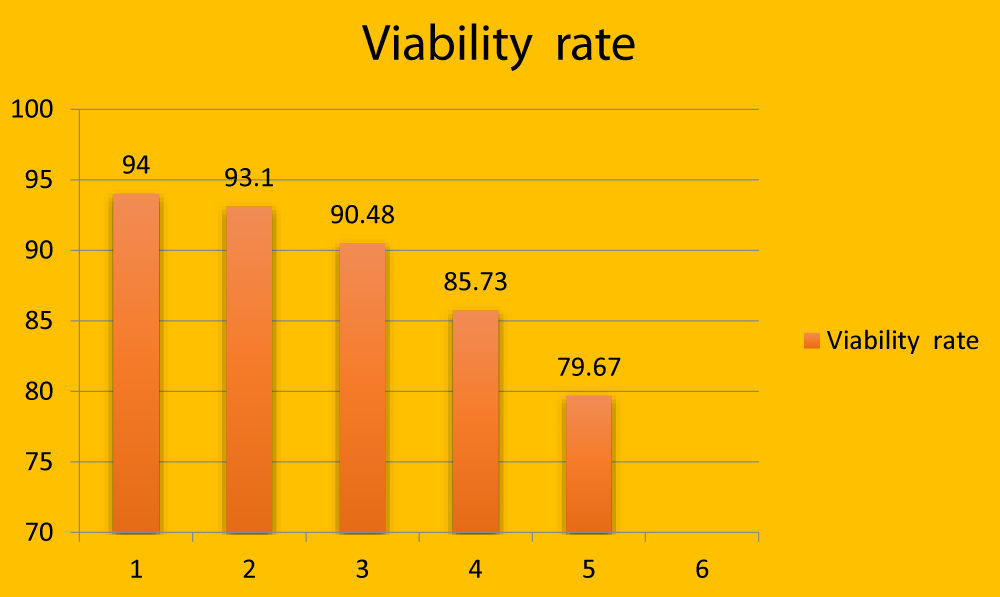
Viability rate (%) (Mean ± SE) |
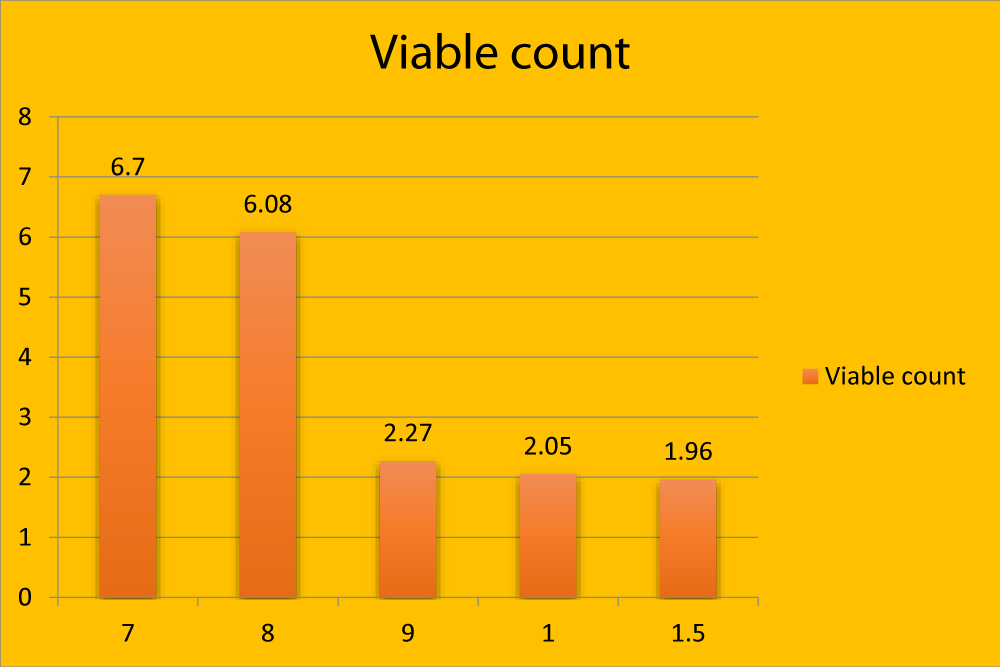
| 7 months | 8.15 ± 0.68a | 6.70 ± 0.06a | 0.69 ± 0. 28a | 94.00 ± 1.17a |
| 8 months | 6.55 ± 0.26a | 6.080 ± 0.26a | 0.62 ± 0.27a | 93.10 ± 0.89a |
| 9 months | 4.85 ± 0.42b | 2.27 ± 0.19b | 1.94 ± 0.30b | 90.48 ± 5.28b |
| 1 year | 3.90 ± 0.34b | 2.05 ± 0.05b | 2.10 ± 0. 28b | 85.73 ± 4.60b |
| 11/2 year | 3.51 ± 0.43b | 1.96 ± 0.23b | 2.57 ± 0. 42b | 79.67 ± 2.90b |
| (Values within a column with different superscripts differ significantly from each other at p < 0.05). | ||||
Figure 1: Graphical representation showing total cell yield (in million) in different age groups.
Figure 2: Graphical representation showing viable count (in million) in different age groups.
Figure 3: Graphical representation showing dead count (in million) in different age groups.
Figure 4: Graphical representation showing viability rate (in millions) in different age groups.
Statistical analysis of data
All data were analyzed using suitable statistical analysis. For cell yield, the Kolmogorov Smirnov test was performed to assess the normality of variables. For parametric variables, ANOVA was used to compare the difference between the groups. For non-parametric variables, the Kruskal Wallis test was used to compare the difference between the groups. All analysis was done by using SPSS-16. Statistical calculations (mean ± standard error) were recorded according to the standard statistical procedures recommended by Snedecor and Cochran, 1994 [4].
Cell viability and cell yield
In the present study, the viability of the cell was evaluated by the trypan blue exclusion method using a haematocytometer. Trypan blue has a high affinity to nuclear DNA, which traverse the membrane in a dead cell and dye it blue. Hence, its absence demonstrated that the cells were intact and live and counted to maintain the seeding density in culture as per Eca, et al. [5].
In the present study, the cell yield of AD-MSCs was 8.15 ± 0.68, 6.55 ± 0.26, 4.85 ± 0.42, 3.90 ± 0.34, and 3.51 ± 0.43 in different age groups 7, 8,9 months,1 year and 1½ year respectively.
The results indicated that in younger age groups, cell yield and viability percentage were more than in animals of more than one year of age. In younger age groups proliferation rate was found to be more when compared to adults as reported by Scheube, et al. [5], Tokalov, et al. [7], and Choudhery, et al. [8]. These observed that adipose tissue harvested from aged donors yielded the lowest number of total nucleated cells. Cell viability of the SVF, as determined by Trypan blue exclusion, showed that the cells per gram of adipose tissue decreased as age advanced. In this study, AD-MSCs were able to form colonies in all the age groups, however, an age-related decrease in CFUs was observed by Choudhery, et al. [8]. The number of CFUs was found to be inversely proportional to the donor age.
In the present study, the size of CFUs and the number of cells in each CFUs were found to be increased as culture advances in all age groups. This indicated the active proliferation of fAD-MSCs in culture. This is in contrast to Choudhery, et al. [8] in humans, who observed that the size of the CFUs was found to be more in a sample collected from aged donors. This indicated the poor functionality of MSCs isolated from aged donors. Based on the results of this study, of fAD-MSCs isolated from seven months to 1½ years of age might be anticipated to be more useful cell sources for tissue engineering and regeneration medicine application.
Luna, et al. [9] in mice of 30, 90, and 120 days the yield, morphology, and viability of AD-MSCs differ according to age.
Chen, et al. [10] and Wu, et al. [11] observed higher density and greater multipotentiality of AD-MSCs harvested at a younger age in humans. Kakudo, et al. [12] reported in humans prolonged digestion with collagenase may reduce cell viability. According to Hepshiba, et al. (2011), trypsin digestion in buffalo adipose tissue yield was reduced Ethics approval and consent to participate: Omental adipose tissue was collected. Favorable ethical approval was given by the Institutional Committee for stem cell research and therapy. Tamil Nadu Veterinary and animal science. Having agenda No. 04/2016.
Consent to publish: All authors have given consent to publish the work.
Authors’ contributions: Shazia N has conducted the research, analyzed, interpreted the data, and contributed to writing the manuscript. Geetha R, TA. Kannan, and Sabiha hayath analyzed the data and corrected the manuscript and S. Hussain collected the clinical samples. All authors read and approved the final manuscript.
Funding: Centre for Stem cell Research and Regenerative Medicine, Madras Veterinary College, Chennai.
Availability of data and materials: The material described in the manuscript should be available to any scientist without copying.
The author thanks the Dean and Center for Stem Cell Research and Regenerative Medicine, Madras Veterinary College, Chennai.
- Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, et al. Human Stromal (Mesenchymal) Stem Cells from Bone Marrow, Adipose Tissue and Skin Exhibit Differences in Molecular Phenotype and Differentiation Potential. Stem Cell Rev Rep. 2013; 9: 32–43. PubMed: https://pubmed.ncbi.nlm.nih.gov/22529014/
- Aliborzi G, Vahdati A, Mehrabani B, Hosseini SE. Isolation, characterization and growth kinetic comparison of bone marrow and adipose tissue mesenchymal stem cells of guinea pig. Inter J Stem Cells. 2016; 9: 115-123. PubMed: https://pubmed.ncbi.nlm.nih.gov/27426093/
- Archana M, Basha SH, Kannan TA, Mangalagowri A, William BJ, et al. Differentiation potential of ovine bone marrow derived mesenchymal stem cells. Int J Adv Res. 2015; 3: 1554-1557.
- Snedecor GM, Cochran WG. In: Statistical Method.8th edn; Oxford and IBN Publishing Company, New Delhi, India. 1994.
- Eca LP, Raalho RB, Oliveira IS, Gomes PO, Pontes P, et al. Comparative study of technique to obtain stem cells from bone marrow collection between the iliac crest and the femoral epiphysis in rabbit. Acta Cir Bras. 2009; 24: 400-404. PubMed: https://pubmed.ncbi.nlm.nih.gov/19851694/
- Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003; 42: 2073-2080. PubMed: https://pubmed.ncbi.nlm.nih.gov/14680729/
- Tokalov SV, Gruner S, Schindler S, Wolf G, Baumann M, et al. Age related changes in the frequency of mesenchymal stem cells in bone marrow of rats. Stem Cells Dev.2003; 16: 439-446. PubMed: https://pubmed.ncbi.nlm.nih.gov/17610374/
- Choudhery MS, Badowski M, Muise A, Dierce JP, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014; 12: 1-144. PubMed: https://pubmed.ncbi.nlm.nih.gov/24397850/
- Luna ACL, Madeira MEP, Conceicao TO, Moreira JALC, Laiso RAN et al. Characterization of adipose-derived stem cells of anatomical region from mice. Bio Med Central Res Notes. 2014; 7: 1-12. PubMed: https://pubmed.ncbi.nlm.nih.gov/25138545/
- Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012; 16: 582-593. PubMed: https://pubmed.ncbi.nlm.nih.gov/21545685/
- Wu W, Niklason L, Steinbacher DM. The effect of age on human adipose-derived stem cells. Plast Reconstr Surg. 2013; 131: 27-37. PubMed: https://pubmed.ncbi.nlm.nih.gov/22965240/
- Kakudo N, Naoki M, Takeshi O, Kenji K. Potential of adipose-derived stem cells for regeneration medicine; clinical application and usefulness of fat grafting. Stem Cell Ther. 2014; 4: 1-3.