More Information
Submitted: June 01, 2022 | Approved: June 09, 2022 | Published: June 10, 2022
How to cite this article: Santinelli E, Cerretti R, De Angelis G, Mariotti B, Ciangola G, et al. Allogeneic hematopoietic cell transplantation to treat two synchronous hematologic malignancies. J Stem Cell Ther Transplant. 2022; 6: 005-007.
DOI: 10.29328/journal.jsctt.1001025
Copyright License: © 2022 Santinelli E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Allogeneic hematopoietic cell transplantation; Synchronous hematologic malignancies; Conditioning regimen; Graft versus tumor
Allogeneic hematopoietic cell transplantation to treat two synchronous hematologic malignancies
Enrico Santinelli*, Raffaella Cerretti, Gottardo De Angelis, Benedetta Mariotti, Giulia Ciangola, Camilla Page, Elisa Lindfors Rossi, Gianmario Pasqualone and William Arcese
Rome Transplant Network, Department of Hematology, Stem Cell Transplant Unit, Tor Vergata University, Rome, Italy
*Address for Correspondence: Enrico Santinelli, Rome Transplant Network, Department of Hematology, Stem Cell Transplant Unit, Tor Vergata University, Rome, Italy, Email: [email protected]
Allogeneic hematopoietic cell transplantation often represents the only solution for several poor-prognosis hematologic malignancies. The curative strategy for patients with synchronous hematologic disorders is always difficult and, in most cases, ineffective. Herein, we report an unusual case of synchronous hematologic disorders successfully treated with an “ad-hoc” conditioning regimen followed by allogeneic hematopoietic cell transplantation.
The coexistence of two hematological malignancies represents a clinical dilemma for hematologists and hematopoietic cell transplantation (HCT) appears to be often the only potential solution in eligible patients. Furthermore, this approach appears to be more valuable when the myelodysplastic syndrome is one of the concurrent diseases, as allogeneic-HCT is the exclusive curative strategy in this condition [1].
Here we report a case where allogeneic HCT was performed to treat two hematological disorders and its post-transplant long-term disease monitoring.
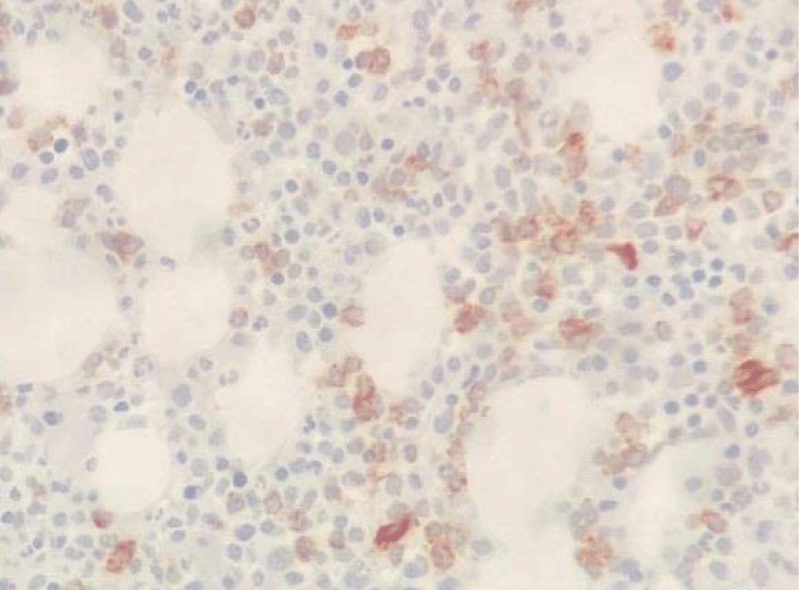
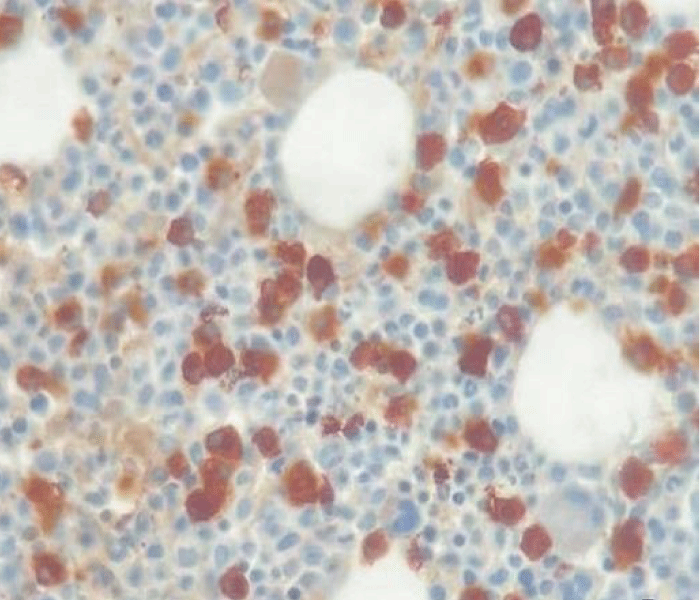
In May 2017, a 44-year-old-woman presented with persistent mild neutropenia, with normal hemoglobin and platelet count. Peripheral blood smear showed a 2% circulating blasts. The bone marrow aspirate showed multi-lineage dysplasia with 12% of CD34+ and CD117+ myeloblasts assessed by flow cytometry and, in absence of myeloma-defining events, an 18% plasma-cell infiltrate. The bone marrow biopsy confirmed the morphological assessment (Figures 1,2). Cytogenetic examination showed a normal female karyotype with no translocations. Serum protein electrophoresis immunofixation displayed an IgA-k monoclonality with a 65,51 k/λ free light chain ratio, also Bence Jones proteinuria resulted positive.
Figure 1: CD34 Immunostaining (20x).
Figure 2: Kappa Immunostaining (40x).
The final diagnosis concluded for myelodysplastic syndrome with an excess of blasts type 2 (MDS-EB type 2, IPSS-intermediate 2) [1] with associated smoldering myeloma.
As no HLA-identical sibling was available, the search for an unrelated donor through the Bone Marrow Donor Registry identified a 30 - year-old female donor who was 10/10 HLA matched with the recipient. Meanwhile, in order to treat the myelodysplastic syndrome and wishing for a possible additional effect on plasma-cell disorder [2], subcutaneous Azacitidine at a dose of 75 mg/m2 for 7 days every 28 days was started. After five courses of therapy with a hypomethylating agent, the BM aspirate re-evaluation displayed a reduction of both blastic and plasma-cell infiltrates to 8.5% and 10% respectively, with multi-lineage dysplasia persistence.
On January 2018, the patient underwent allogeneic-HCT with an “ad hoc” conditioning regimen consisting of Melphalan 140 mg/m2 IV on day - 8, Thiotepa 5 mg/kg IV on day -6, Busulfan 3,2 mg/kg IV on days - 5 and - 4 and Fludarabine 50 mg/m2 IV from day -5 to day -3 (M-TBF). At day 0 1.8 × 106/kg CD34+ cells harvested from bone marrow (according to the donor choice) were infused. The graft versus host disease (GvHD) prophylaxis consisted of a calcineurin inhibitor (cyclosporine A) 3 mg/kg in continuous infusion from day -1, rabbit anti-human T-lymphocyte immunoglobulin (ATG-Fresenius) 5 mg/kg from day - 4 to day - 1 and methotrexate 15 mg/m2 on day + 1 and 10 mg/m2 on days + 3, + 6 and + 11.
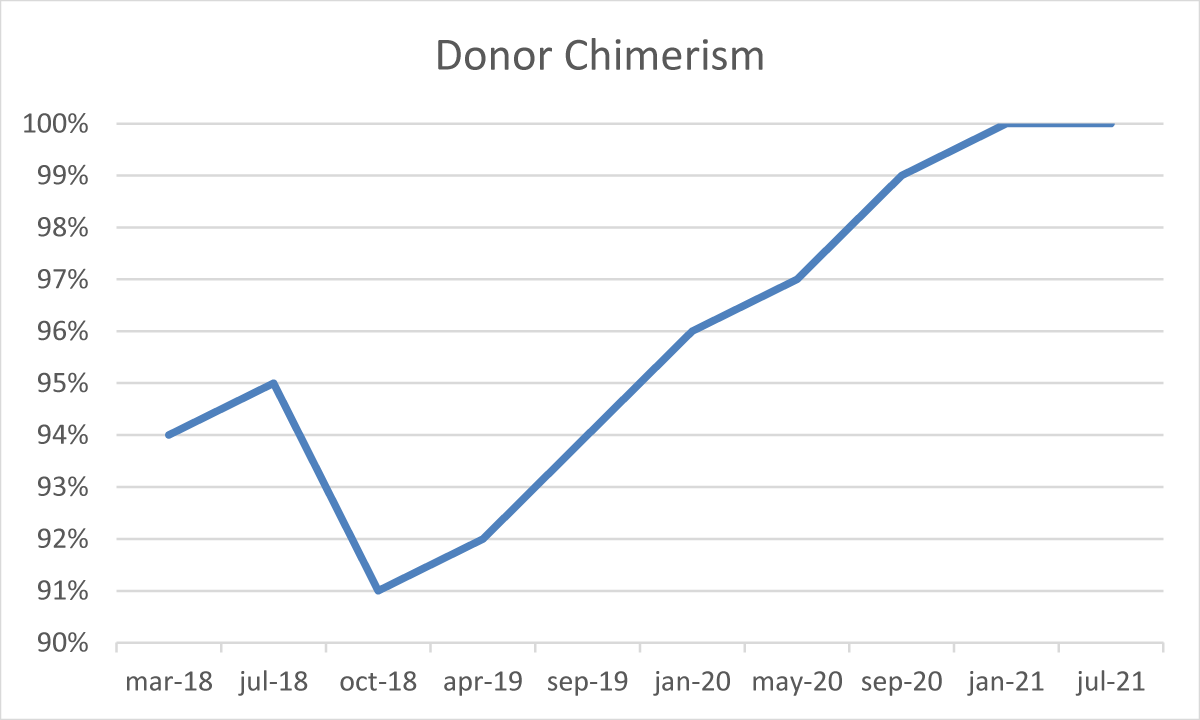
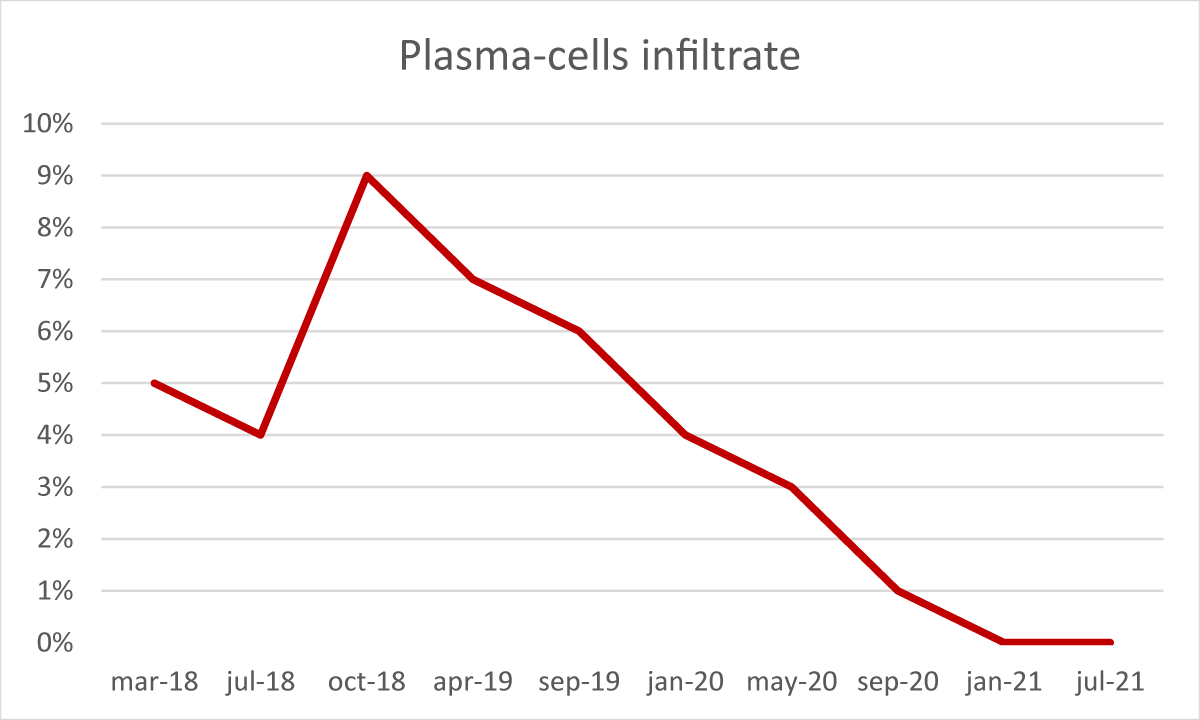
After the transplant, the patient developed a skin-limited grade I GvHD which resolved spontaneously. From day + 50 cyclosporine a tapering was started. In absence of any other GvHD sign, cyclosporine A was discontinued after six months. During the post-transplant follow-up, several bone marrow examinations and chimerism assessments by STR sequencing were performed [3]. The morphological analysis demonstrated complete recovery of hematopoiesis, with no blasts or signs of dysplasia, but with the persistence of a minor IgA-positive plasma-cell infiltrate (5% - 9%). The STR sequencing evaluation constantly showed mixed chimerism ranging from 91% to 96% cells of donor origin. Furthermore, serum and urinary immunofixation still resulted positive and the serum k/λ free light chain ratio was still altered (10,5).
From January 2019, we observed a gradual reduction of BM plasma-cells infiltrate associated with a concomitant and progressive reduction of recipient chimerism fraction, reaching the absence of plasma-cell infiltrate (Figure 3) and full-donor chimerism (Figure 4) in January 2021, confirmed in July 2021.
Figure 3: Donor chimerism during follow-up
Figure 4: Plasma-cell infiltrate during follow-up
In January 2021 Bence Jones proteinuria assessment resulted negative for the first time (confirmed in July and November 2021), however, serum immunofixation continued to be positive but the k/λ free light chain ratio kept improving (6,7 in November 2021).
At the moment we are waiting to reassess the patient’s disease status expecting to confirm the full donor chimerism, the absence of medullary plasma-cell infiltrate and hoping for a further k/λ free light chain ratio reduction.
The simultaneous diagnosis of two hematological disorders is rather unusual and mostly assessed in elderly patients; in this population therapeutic options are limited and the prognosis is consequentially adverse [4,5]. Few data are available for the younger population, however, the access to more intensive treatments may improve the outcome.
In these cases, the best therapeutic strategy is not easy to plan, and, if feasible, front-line allogeneic-HCT seems to be a reasonable option. To our knowledge, only one retrospective study analyzing outcomes of concurrent myelodysplastic syndromes and lymphoid malignancies at the time of transplant was recently conducted. In this analysis Zimmerman, et al. proved that allogeneic-HCT was effective in terms of overall response rate, however, global relapse incidence was high, suggesting that a prior treatment must be considered [6]. Few cases of synchronous multiple myeloma and myelodysplastic syndrome harboring del (5q) have been successfully brought to HCT with lenalidomide [7,8].
In our case, we chose to start an MDS-specific therapy accordingly to the “non-treatment need” of smoldering myeloma. Nevertheless, we decided to add Melphalan to a classic TBF reduced-intensity conditioning regimen considering the plasma-cells sensitivity to nitrogen mustards.
After transplantation, maintenance therapy with Lenalidomide was considered but not chosen, preferring its utilization as part of combination treatment in case of symptomatic myeloma evolution.
The progressive plasma-cell infiltrate reduction seen after transplantation, demonstrates the graft-versus-myeloma effect and its independence from GvHD manifestations [9]. Moreover, the mixed chimerism persistence represented a possible donor-lymphocyte infusion (DLI) indication [10], however, we decided not to proceed with DLI due to GvHD induction risk [11,12] compared to the achievement of remission in a “non-treatment need” condition [13]. However, in case of future evolution in symptomatic myeloma, DLI would be useful to consolidate the response obtained through conventional treatments.
On one hand, this case shows the complex management of two synchronous hematological disorders due to the lack of bridge-to-transplant uniformed strategies. On the other hand, our experience displays the pivotal role of allogeneic-HCT in this challenging setting, particularly through the graft-versus-tumor effect.
- Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020 Nov;95(11):1399-1420. doi: 10.1002/ajh.25950. PMID: 32744763.
- Khong T, Sharkey J, Spencer A. The effect of azacitidine on interleukin-6 signaling and nuclear factor-kappaB activation and its in vitro and in vivo activity against multiple myeloma. Haematologica. 2008 Jun;93(6):860-9. doi: 10.3324/haematol.12261. Epub 2008 Apr 28. PMID: 18443271.
- Abatay-Sel F, Savran-Oguz F, Kalayoglu-Besisik S, Mastanzade M, Duvarci-Ogret Y, Yonal-Hindilerden I, Aydin F. Short Tandem Repeat-Polymerase Chain Reaction (STR-PCR) with Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) Method Using for Chimerism Analysis. Clin Lab. 2019 Sep 1;65(9). doi: 10.7754/Clin.Lab.2019.190221. PMID: 31532093.
- Mufti GJ, Hamblin TJ, Clein GP, Race C. Coexistent myelodysplasia and plasma cell neoplasia. Br J Haematol. 1983 May;54(1):91-6. doi: 10.1111/j.1365-2141.1983.tb02070.x. PMID: 6849839.
- Florensa L, Vallespí T, Woessner S, Domingo A, Crespo N, Rozman M, Aguilar JL, Irriguible D, Zarco A, Millá F, Feliu E. Incidence and characteristics of lymphoid malignancies in untreated myelodysplastic syndromes. Leuk Lymphoma. 1996 Nov;23(5-6):609-12. doi: 10.3109/10428199609054871. PMID: 9031093.
- Zimmerman Z, Scott BL, Gopal AK, Sandmaier BM, Maloney DG, Deeg HJ. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and concurrent lymphoid malignancy. Bone Marrow Transplant. 2012 Jun;47(6):804-9. doi: 10.1038/bmt.2011.180. Epub 2011 Sep 12. PMID: 21909142; PMCID: PMC3237793.
- Ortega M, Mallo M, Solé F, Sánchez-Morata C, López-Andreoni L, Martínez-Morgado N, Gironella M, Valcárcel D, Vallespí T. 5q- syndrome and multiple myeloma diagnosed simultaneously and successful treated with lenalidomide. Leuk Res. 2013 Oct;37(10):1248-50. doi: 10.1016/j.leukres.2013.06.021. Epub 2013 Jul 26. PMID: 23891188.
- Nishihori T, Komrokji R, Shain K, Anasetti C. Allogeneic hematopoietic cell transplantation for concurrent multiple myeloma and myelodysplastic syndrome. Bone Marrow Transplant. 2015 Feb;50(2):296-7. doi: 10.1038/bmt.2014.233. Epub 2014 Oct 20. PMID: 25330222.
- Mehta J, Singhal S. Graft-versus-myeloma. Bone Marrow Transplant. 1998 Nov;22(9):835-43. doi: 10.1038/sj.bmt.1701459. PMID: 9827810.
- Gröger M, Gagelmann N, Wolschke C, von Pein UM, Klyuchnikov E, Christopeit M, Zander A, Ayuk F, Kröger N. Long-Term Results of Prophylactic Donor Lymphocyte Infusions for Patients with Multiple Myeloma after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018 Jul;24(7):1399-1405. doi: 10.1016/j.bbmt.2018.04.018. Epub 2018 Apr 21. PMID: 29684563.
- Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008 Jun;21(2):205-22. doi: 10.1016/j.beha.2008.02.007. PMID: 18503987; PMCID: PMC2504712.
- Scarisbrick JJ, Dignan FL, Tulpule S, Gupta ED, Kolade S, Shaw B, Evison F, Shah G, Tholouli E, Mufti G, Pagliuca A, Malladi R, Raj K. A multicentre UK study of GVHD following DLI: rates of GVHD are high but mortality from GVHD is infrequent. Bone Marrow Transplant. 2015 Jan;50(1):62-7. doi: 10.1038/bmt.2014.227. Epub 2014 Oct 13. PMID: 25310308.
- Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010 Jun;24(6):1121-7. doi: 10.1038/leu.2010.60. Epub 2010 Apr 22. PMID: 20410922; PMCID: PMC7020664.