More Information
Submitted: September 20, 2022 | Approved: September 28, 2022 | Published: September 29, 2022
How to cite this article: Al-Anazi KA, Mutahar E, Abduljalil O, Kanfer S, Kaloyannidis P, et al. The outcome of autologous hematopoietic stem cell transplantation in patients with multiple myeloma. The experience of King Fahad Specialist Hospital in Dammam, Saudi Arabia J Stem Cell Ther Transplant. 2022; 6: 019-028.
DOI: 10.29328/journal.jsctt.1001027
Copyright License: © 2022 Al-Anazi KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Multiple myeloma; Autologous hematopoietic stem cell transplantation; Non-cryopreservation; Outpatient transplantation
The outcome of autologous hematopoietic stem cell transplantation in patients with multiple myeloma. The experience of King Fahad Specialist Hospital in Dammam, Saudi Arabia
Khalid Ahmed Al-Anazi1*, Mutahar E1, Abduljalil O1, Kanfer S1, Kaloyannidis P1, Estanislao A1, Apostolidis I1, Almokhtar N1, Darweesh M1, Abdulbaqi M1, Alenazi W1, Alshammasi Z1, Alshaibani Z1, Kawarie M1, Raslan H2, Albahrani A3, Alsaber A2, AlMulhem N3, Dridi W4, Aldayel A2, Alrabeh R2, Alshami A1, Ayyad A1, Abu Rahma F1, Lardizabal J1, Salam A3, Haque K3, Alsagheir A1 and Alhashmi H1
1Department of Hematology and Hematopoietic Stem Cell Transplantation, Oncology Center, King Fahad Specialist Hospital, PO Box: 15215, Dammam 31444, Saudi Arabia
2Hematopathology and Flowcytometry, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
3Apheresis and Blood Bank, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
4Cytogenetics, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
*Address for Correspondence: Dr. Khalid Ahmed Al-Anazi, Consultant Hemato-Oncologist, Department of Hematology and Hematopoietic Stem Cell Transplantation, Oncology Center, King Fahad Specialist Hospital, PO Box: 15215, Dammam 31444, Saudi Arabia, Email: [email protected]
Background: Aautologous hematopoietic stem cell transplants (HSCT) is the standard of care for newly diagnosed patients with multiple myeloma (MM) who are eligible for autologous transplantation. Although cryopreservation is routinely employed, autologous HSCT can be performed using non-cryopreserved stem cells.
Methods and materials: A retrospective study of patients with MM who received autologous HSCT between the 10th of October 2010 and the 31st of January 2022 at King Fahad Specialist Hospital (KFSH) in Dammam, Saudi Arabia was performed.
Results: Over 11 years and 113 days, a total of 135 autologous HSCTs were performed for 119 patients with MM at our institution. Single autologous HSCTs were performed for 119 patients, while 16 of these patients received either planned tandem autologous transplants or second autografts due to either progression or relapse of their myeloma. The median age of patients with MM at autologous HSCT was 51.5 years. At presentation of their MM, the following high-risk (HR) features were encountered: stage III disease according to the revised international scoring system (RISS) in 12.3%; adverse cytogenetics in 31.93% of patients; advanced bone disease in 60.50%; and renal dysfunction or failure in 11.76% of patients.
A total of 104 autologous HSCTs (77.04%) were performed without cryopreservation while 31 autografts (22.96%) were performed using cryopreserved apheresis stem cell products. Additionally, 54 autologous HSCTs (40.00%) were done at outpatient while 81 autografts (60.00%) were performed in an inpatient setting. Survival for 100 days post-HSCT for all patients with MM who received autologous transplants including those done at outpatient was 100%. The 4 years overall survival (OS) an progression-free survival (PFS) for patients with MM who received non- cryopreserved or fresh autologous HSCTs were 82% and 68% respectively.
Conclusion: Autologous HSCT without cryopreservation is safe, and feasible and can lead to short-term as well as long-term outcomes that are comparable to autologous transplantation with cryopreservation. Non- cryopreserved autologous grafts allow the performance of autologous transplants in an outpatient setting to save beds and reduce costs.
MM, the second commonest hematologic malignancy, is characterized by the proliferation of monoclonal plasma cells in the bone marrow, production of monoclonal proteins, and occurrence of secondary end-organ damage [1-7]. MM is a disease of old age with the median age at diagnosis ranging between 65 and 74 years in the United States of America (USA) and Europe [2,3,8,9]. The global 5 years survival has more than doubled over the past 2 decades due to the availability of several lines of the novel therapeutic agents and HSCT, the recent advancements in diagnostic techniques, and the general improvement in health care [9-11]. The definition of HR-MM implies the presence of any of the following: stage III disease according to the RISS including high serum levels of β-microglobulin and lactate dehydrogenase as well as HR cytogenetics such as del(17p), t(4;14), t(14;16) and chromosome 1 abnormalities; plasma cell leukemia; extramedullary disease; and renal failure [5-9]. In patients with MM who are eligible for autologous HSCT, 3-4 cycles of induction therapy that consists of either bortezomib, lenalidomide, dexamethasone (VRd) or bortezomib, cyclophosphamide, dexamethasone (VCd), or bortezomib, thalidomide, dexamethasone (VTd) are usually given followed by single autologous HSCT [5,8,9]. However, in patients with HR disease it is recommended to give induction therapy with either daratumumab, bortezomib, lenalidomide, dexamethasone (Dara-VRd) or carfilzomib, lenalidomide, dexamethasone (KRd) as alternatives to VRd followed by single or tandem autologous HSCT [5,8].
In autologous HSCT, cryopreservation of hematopoietic stem cells is routinely employed. The standard and the most commonly used cryopreservative is dimethyl sulfoxide (DMSO) which prevents freezing damage to living cells [3,7]. DMSO is generally safe and nontoxic but its use is associated with significant side effects that include nausea, vomiting, abdominal cramps, hemolysis, as well as systemic adverse reactions. After cryopreservation and thawing of stem cells, 20% - 30% of the collected stem cells become nonviable due to early irreversible apoptosis [3].
A large number of studies from various parts of the world and one meta-analysis have shown that non-cryopreserved autologous HSCT for MM is not only simple, safe, and cost-effective but also can give results that are at least equivalent to autologous HSCT with cryopreservation [3,7]. This retrospective study was carried out to explore the short-term as well as the long-term outcomes of patients with MM subjected to autologous HSCT, particularly those receiving non-cryopreserved autologous grafts.
A retrospective study was conducted between the the 10th of October 2010 and the 31st of January 2022. The medical records, clinical data as well as laboratory data of all patients with MM who received autologous HSCT at KFSH in Dammam, Saudi Arabia during the time period specified above were retrieved for analysis. For our cryopreserved autologous HSCTs, after controlling the primary disease using certain induction therapeutic regimens, mobilization of stem cells was performed using cyclophosphamide and filgrastim, then collection of mobilized stem cells by apheresis was done followed by cryopreservation of the apheresis product. After administration of high-dose (HD) melphalan, the cryopreserved stem cells were infused after thawing. However, for non-cryopreserved autologous grafts, the same process was followed with the exception of keeping the collected stem cells at 4º C for 24 to 48 hours instead of cryopreserving them. Then the fresh stem cells were infused within 24 hours after administration of HD melphalan.
During stem cell mobilization, once the CD34+ cell count in peripheral blood exceeded 10.0 to 20.0 × 106/kg body weight, stem cell collection by leukapheresis was usually commenced. We aimed to obtain a target of 3.0 to 4.0 × 106 CD34+ cells/kg in case a single autologous HSCT was desired and a target of 6.0 to 8.0 × 106 CD34+ cells/kg in case a tandem transplant was planned. After day 0 of autologous HSCT, prophylactic antimicrobials were administered, and starting from day 5 post-HSCT till the day of neutrophil engraftment daily doses of filgrastim were administered.
Statistical analysis
The SSPS version 22 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The Kaplan-Meier method with a log-rank test was used to estimate the survival rates and to identify risk factors that influenced the treatment outcome. OS was defined as the duration from the day of graft infusion until death or the date of the last follow-up for alive patients. PFS was defined as the period from graft infusion till the documentation of disease relapse/progression or last follow-up for the non-relapsed/progressed patients. Time periods of autologous HSCT (2010- 2017 versus 2018-2022) and type of autologous HSCT (inpatient or outpatient basis), were evaluated as factors with potential impact on survival rates (OS and PFS).
During the study period, 11 years and 113 days, a total of 135 autologous HSCTs were performed for 119 patients with MM at KFSH in Dammam, Saudi Arabia. Single autologous grafts were offered to 119 patients. Twelve of these 119 patients received planned tandem autologous HSCTs while four other patients received second autologous grafts due to relapse or progression of their MM after receiving appropriate salvage therapies. Out of the 119 patients, there were 61 females and 58 males and the median age of patients at HSCT was 51.5 years. At the presentation of their myeloma, the following HR features were encountered: stage III disease according to the RISS in 12.3% of patients, adverse cytogenetics in 31.93% and extensive bone involvement in 60.50% of patients, while 11.76% of patients had either renal dysfunction or end-stage renal disease (ESRD) (Tables 1-4).
| Table 1: Stage of disease in patients with MM according to the revised international staging system (RISS) subjected to Auto-HSCT. | ||
| Stage | Number | Percentage |
| I | 52 | 43.7 |
| II | 41 | 34.5 |
| III | 22 | 12.3 |
| Unknown | 4 | 3.7 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
| Table 2: Cytogenetic abnormalities in patients with MM subjected to Auto-HSCT. | ||
| Cytogenetic abnormality | Number | Percentage |
| Normal | 38 | 31.93 |
| Chromosome 3 abnormalities including deletion of chromosome 3 |
12 | 10.08 |
| 17 p Deletion | 10 | 8.40 |
| Translocation of chromosome 14 including: t 4,14; t 6,14; t 14,16; t 14,20 |
19 | 15.97 |
| Trisomies of chromosomes: 3,7,9,15,17 | 15 | 12.60 |
| Monosomies of chromosomes: 13; 16 | 9 | 7.56 |
| Not available; Unknown | 16 | 13.45% |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
| Table 3: Bone lesions in patients with MM subjected to Auto-HSCT. | ||
| Type of Bone Involvement | Number | Percentage |
| Localized or single lytic lesion (s) | 20 | 16.81 |
| Multiple lytic lesions | 60 | 50.42 |
| Pathological fractures requiring surgery | 12 | 10.08 |
| Osteopenia | 43 | 36.13 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
| Table 4: Renal dysfunction in patients with MM subjected to Auto-HSCT. | ||
| Type of renal dysfunction | Number | Percentage |
| End stage renal disease (ESRD) on hemodialysis [serum creatinine: 629 -1328] Creatinine clearance < 10 |
4 | 3.36 |
| ESRD not yet on hemodialysis [serum creatinine: 381- 477] Creatinine clearance: 10-20 |
2 | 1.68 |
| Significant renal dysfunction [serum creatinine: 185 - 204] Creatinine clearance: 20-30 |
8 | 6.72 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
Out of the 135 autologous HSCTs, 104 autologous HSCTs (77.04%) were performed using non-cryopreserved stem cells while 31 autologous grafts (22.96%) used cryopreserved stem cells. Additionally, 54 autologous HSCTs (40.00%) were performed at the outpatient setting while 81 autografts (60.00%) were conducted as inpatient. Out of the 54 autologous HSCTs that were performed at outpatient: in 39 transplants (72.22%) fresh or non-cryopreserved stem cells were used, while in 15 autologous HSCTs (27.78%) cryopreserved stem cells were used.
Regarding the initial therapy administered to the primary disease, 89.9% of our patients received bortezomib-based therapy either the doublet regimen bortezomib and dexamethasone (VD) or a triplet regimen such as VRd and only 9% of patients (7.6%) received more intensive regimens containing PACE chemotherapy (cisplatin, doxorubicin, cyclophosphamide and etoposide) including VTD-PACE, VRd-PACE and KRd-PACE (Table 5). The number of lines of chemotherapy administered before autologous HSCT was as follows: 88 patients (73.9%) received 1 line of therapy, 20 patients (16.8%) received 2 lines of therapy, while 11 patients (9.2%) received ≥ 3 lines of chemotherapy (Table 6). Thirteen patients (9.6%) achieved partial response (PR), 82 MM patients (60.3%) achieved very good PR (VGPR), and 39 patients (28.7%) achieved complete response (CR) while only 2 MM patients (1.5%) achieved stringent CR prior to autologous HSCT (Table 7). Twenty-seven patients (22,7%) received maintenance therapy for 1 to 2 years, 63 patients (52.9%) received maintenance treatment till disease progression, and 29 patients (24.4%) did not receive maintenance therapy (Table 8).
| Table 5: Initial therapy given to patients with MM subjected to Auto-HSCT. | ||
| Regimen/Protocol | Number of patients | Percentage |
| Bortezomib + Dexamethasone [VD] | 21 | 17.6 |
| Bortezomib triplet protocols VTD/VCD/VRd |
86 | 72.3 |
| Lenalidomide + dexamethasone [RD] | 3 | 2.5 |
| More intensive regimens VTD-PACE/VRd-PACE/KRd-PACE |
9 | 7.6 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplan-tation; VCd: bortezomib, cyclophosphamide, dexamethasone; VTd: bortezomib, thalidomide, dexamethasone; VRd: bortezomib, lenalidomide, dexamethasone; VTD-PACE: bortezomib, thalidomide, dexamethasone, cisplatin, adriamycin, cyclophos-phamide, etoposide; KRd: carfilzomib, lenalidomide, dexamethasone. | ||
| Table 6: Number of lines of therapy given to patients with MM prior to Auto-HSCT. | ||
| Number of treatment lines | Number of patients | Percentage |
| 1 | 88 | 73.9 |
| 2 | 20 | 16.8 |
| ≥ 3 | 11 | 9.2 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
| Table 7: Treatment responses achieved in patients with MM prior to Auto-HSCT. | ||
| Type of response | Number of patients | Percentage |
| Partial response [PR] | 13 | 9.6 |
| Very good partial response [VGPR] | 82 | 60.3 |
| Complete response [CR] | 39 | 28.7 |
| Stringent complete response [str. CR] | 2 | 1.5 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
| Table 8: Maintenance therapy administered to patients with MM subjected to Auto-HSCT. | ||
| Given/Not given/Duration | Number of patients | Percentage |
| Given for 1-2 years | 27 | 22.7 |
| Given till disease progression | 63 | 52.9 |
| Not given | 29 | 24.4 |
| MM: Multiple Myeloma; Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation. | ||
Survival at day 100 post-HSCT for all patients with MM who received their autologous HSCTs including those who received their autologous grafts at outpatient was 100%. The median time for neutrophil engraftment with granulocyte-colony stimulating factor (G-CSF) given from the 5th day post-HSCT onwards was 11 days while the median time for platelet engraftment post autologous HSCT was 17 days. The main complications encountered in the early post-transplant period were as follows: mucositis (grades I to III) in 38% of patients, engraftment syndrome (ES) in 24% of patients, while episodes of febrile neutropenia (FN) were encountered in 31% of patients. The manifestations of ES were: fever, green watery diarrhea, and rarely skin eruptions. The main risk factors for ES were: heavily pre-treated patients with MM, second autologous HSCTs and > 6.0 x 106 CD34 + cells/kilogram body weight. However, severe forms of ES developed in 2 heavily pre-treated HR patients. The first patient had capillary leak syndrome while the second patient developed cytomegalovirus colitis. Despite the complications, both patients were treated successfully.
The long-term outcomes of our patients were excellent.
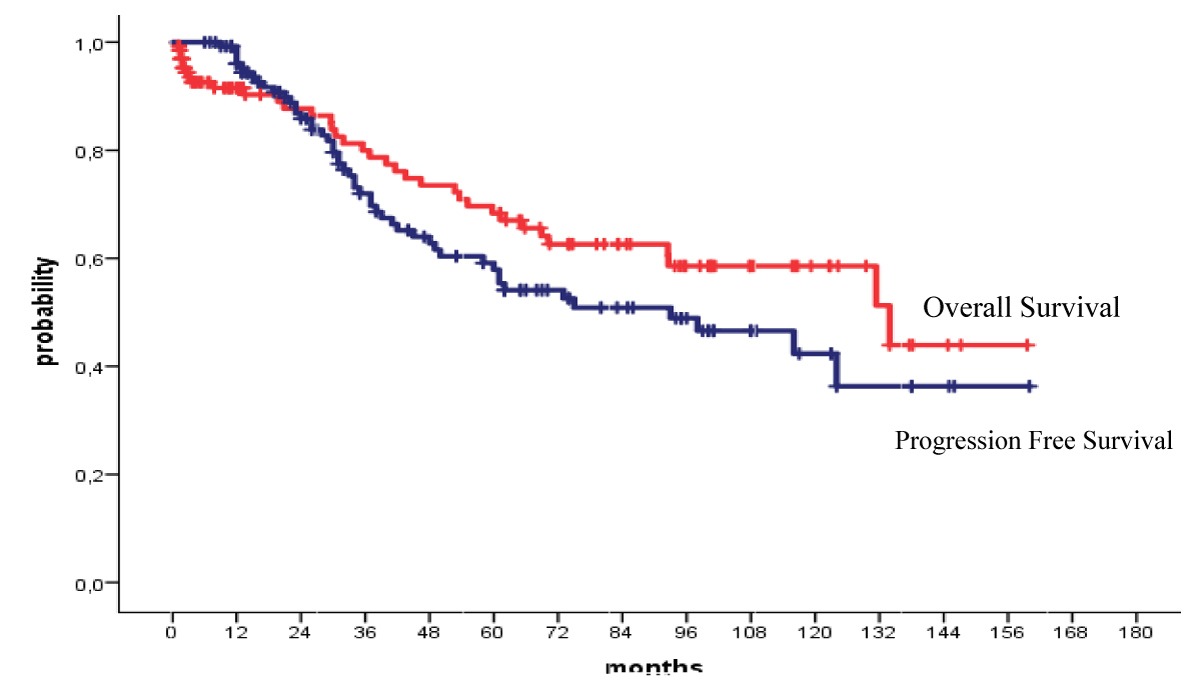
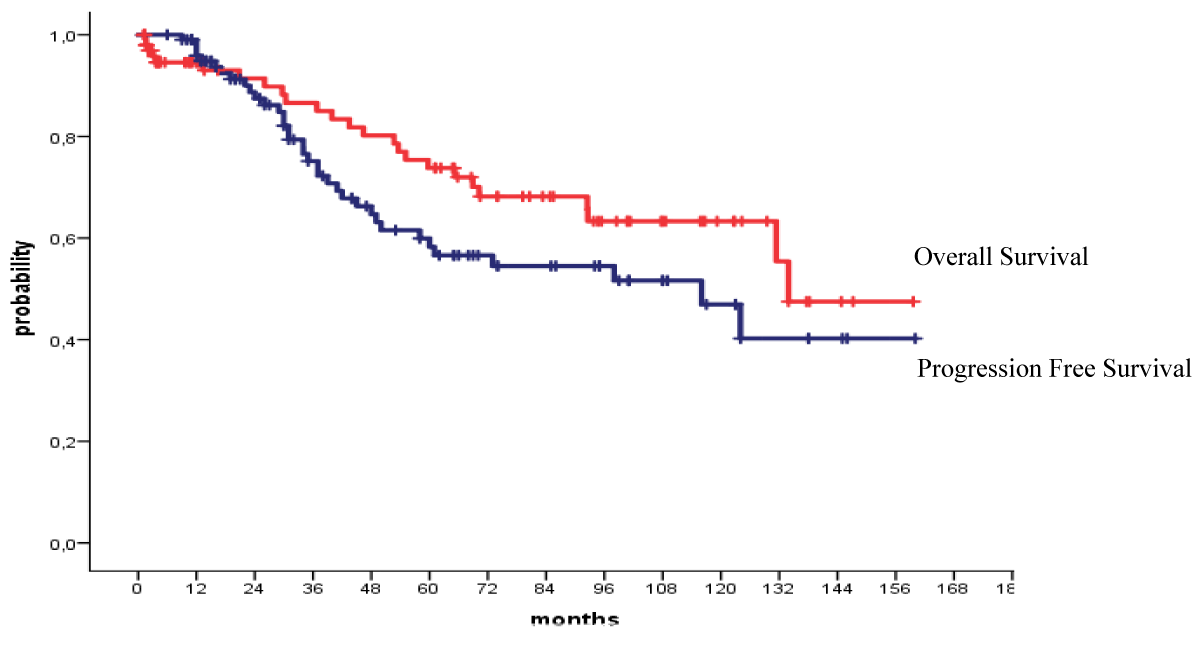
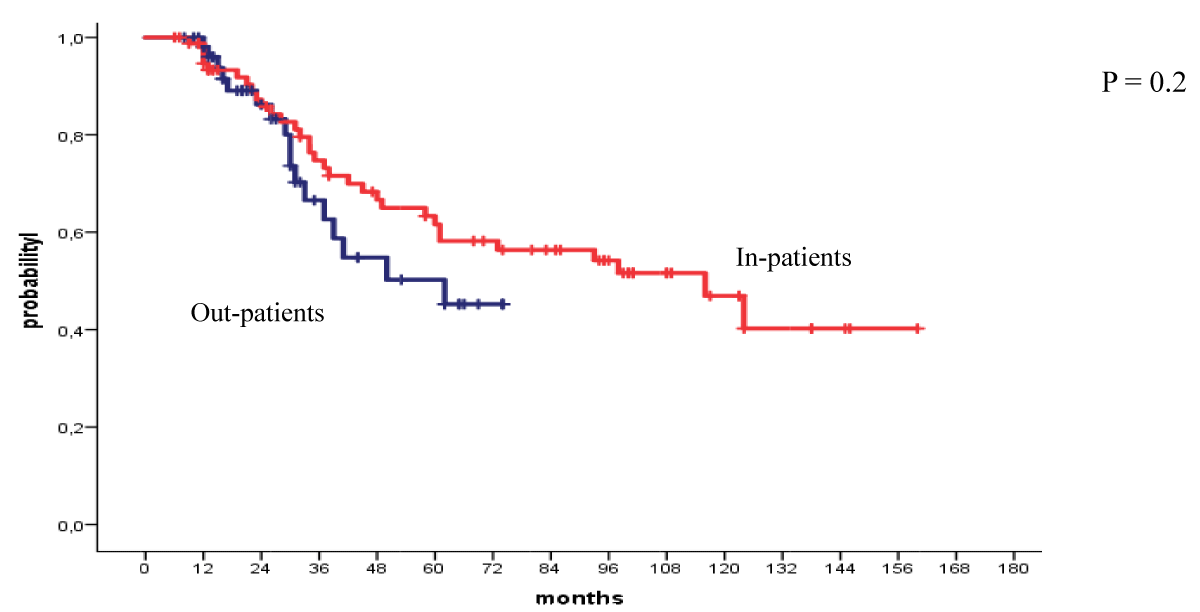
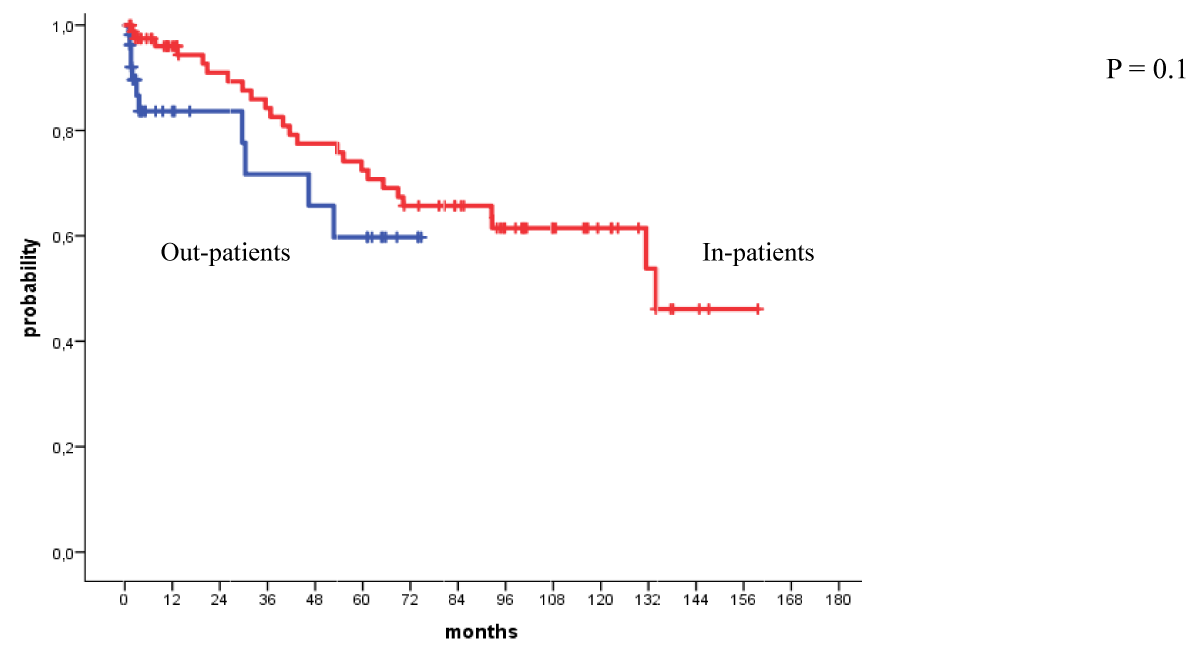
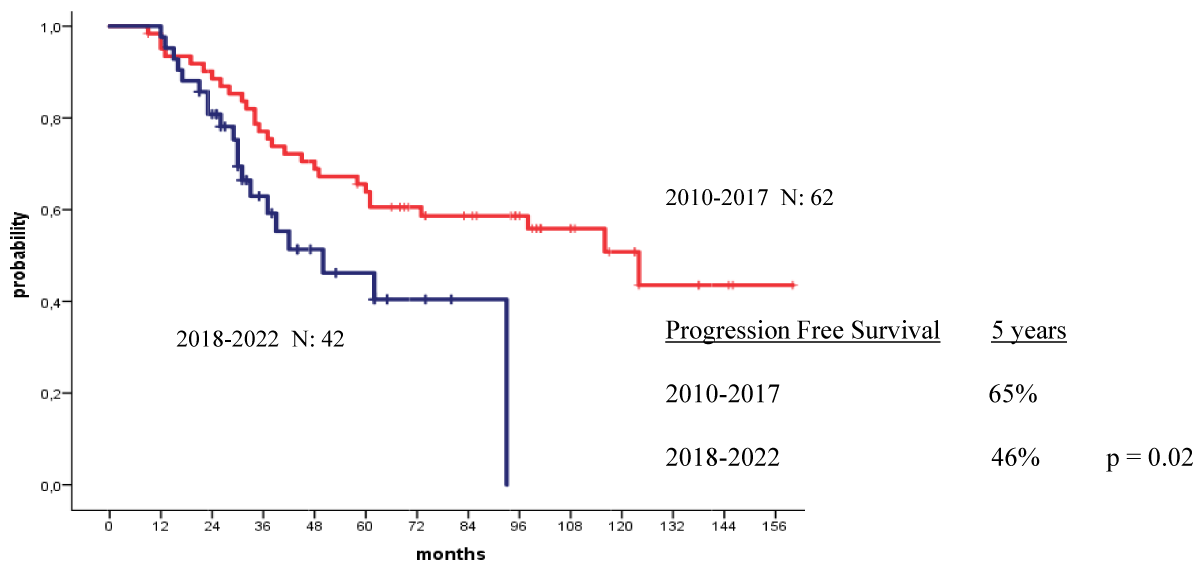
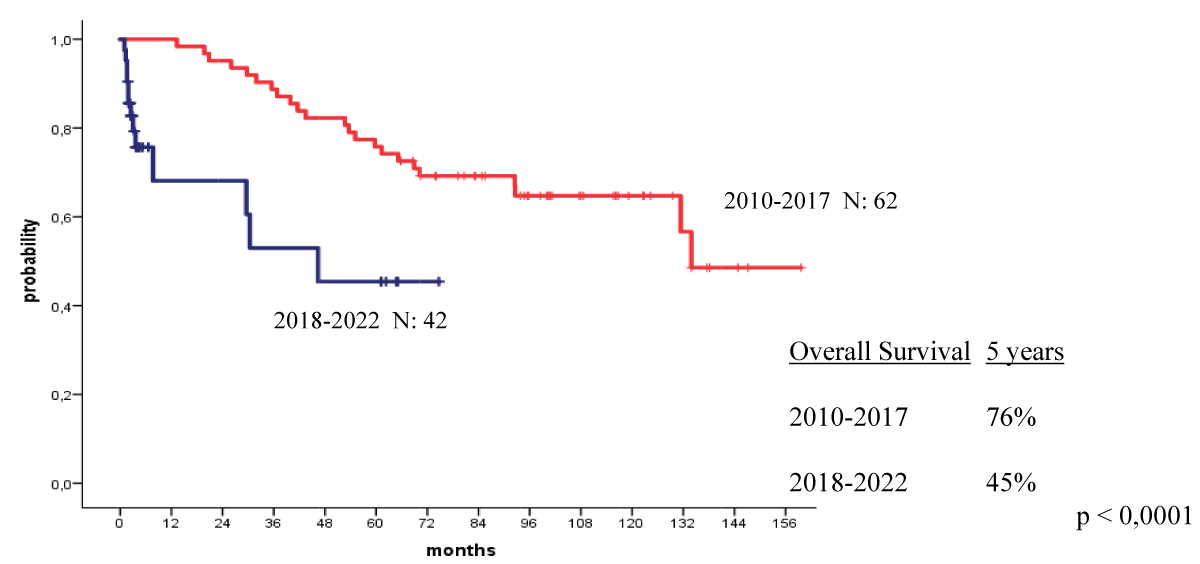
The 4-year OS and PFS for all patients with MM who received their autologous grafts during the study period were 76% and 60% respectively (Figure 1). The 4-year OS and PFS for patients with MM who received non-cryopreserved stem cells, that is fresh grafts, over the same period of time were 82% and 68% respectively (Figure 2). However, there were no significant differences in PFS or OS between patients with MM who received their autografts as an inpatient or as an outpatient (Figures 3,4). Although the 5-year PFS for patients with MM transplanted between 2010 and 2017 was higher than PFS for patients transplanted between 2018 and 2022, the difference was not statistically significant (Figure 5). However, the 5-year OS for patients with MM transplanted between 2010 and 2017 compared to those patients transplanted between 2018 and 2022 showed a difference that was highly significant (Figure 6).
Figure 1: Overall and progression free survival for all patients autografted for multiple myeloma: 2010-2022.
Figure 2: Overall and progression free survival for patients autografted for multiple myeloma with fresh grafts: 2010-2022.
Figure 3: Progression free survival for patients autografted for multiple myeloma by in-or out-patient basis: 2010-2022.
Figure 4: Overall survival for patients autografted for multiple myeloma by in-or out-patient basis: 2010-2022.
Figure 5: Progression free survival for patients autografted for multiple myeloma by the year of transplant: 2010-2017 versus 2018-2022.
Figure 6: Overall survival for patients autografted for multiple myeloma by the year of transplant: 2010-2017 versus 2018-2022.
Since the mid-1990s and despite the recent availability of several lines of novel agents, HD melphalan followed by autologous HSCT is still the standard of care for newly diagnosed patients with MM who are eligible for autologous HSCT [7,12-16]. Eligibility for autologous HSCT is determined by: age, performance status, presence as well as the severity of comorbid medical conditions, and frailty score as frailty has been shown to be a predictor of short survival and is considered an exclusion criterion for autologous HSCT [7,15,17,18]. The long-term outcome of patients with MM subjected to autologous HSCT has improved significantly over the last 3 decades [8,19]. Nishimura KK, et al. reported the long-term outcomes of a total of 4329 patients with newly diagnosed MM treated with autologous HSCT using cryopreserved stem cells at the University of Arkansas in the USA between 1989 and 2014 [19]. The 5 years PFS for the entire population of autologous HSCT recipients had improved from 29% to 68% and the OS for the entire population of autologous HSCT recipients had improved over that time period from 47% to 70% respectively [19]. Cryopreservation of hematopoietic stem cells is routinely employed in the setting of autologous HSCT [3,7,20]. The standard conditioning regimen for patients with MM undergoing autologous HSCT is HD melphalan (200 mg/m2) given intravenously [3,5,7,12,14,15]. However, in patients with renal dysfunction or failure, dose reductions to 100-140 mg/m2 may be needed according to creatinine clearance [5,7]. In patients with MM having renal impairment, several studies have shown that: (1) conditioning therapy with melphalan 140 mg/m2 has an acceptable toxicity and is equally effective to melphalan dose of 200 mg/m2 and (2) melphalan dose adjustment is not indicated in patients having renal failure subjected to autologous HSCT [21-28]. In patients with MM having ESRD receiving hemodialysis, careful evaluation prior to autologous HSCT with the involvement of a multidisciplinary team should be made and dose adjustment for all drugs that adversely affect renal function should be taken into consideration [29,30]. In our study, we did not exclude patients with MM having severe renal dysfunction or even ESRD from having autologous HSCT. Six patients with MM had ESRD, 4 of them were receiving regular hemodialysis and 8 more MM patients having severe renal dysfunction received their autologous HSCTs at our institution. The main differences in the management of these patients compared to those patients with MM having normal renal function were: modifying the doses of medications such as chemotherapy including melphalan conditioning and novel agents according to creatinine clearance. Also, these medications were administered and stem cells were infused after hemodialysis. Melphalan is cleared from plasma and urine in 1 and 6 hours, respectively. Hence, stem cells can be safely infused as early as 8-24 hours following melphalan administration [3,31]. Additionally, studies have indicated that: peripheral blood stem cells can be stored safely at 4°C for at least 5 days, while the patient receives HD chemotherapy; and the viability of stem cells decreases progressively from day 5 onwards [3,32]. Several old and recent studies in addition to one systematic review have shown that autologous HSCT using non-cryopreserved stem cells is simple, safe, cost-effective and leads to short-term as well as long-term outcomes that are at least equivalent to autologous HSCT using cryopreserved stem cells [3,7,20,31,33-37]. The median times of engraftment following non-cryopreserved autografts were 9-14 days for neutrophils and 13-25 days for platelets [3,31,34]. Additionally, treatment-related mortality (TRM) at day 100 post-HSCT using non- cryopreserved autologous stem cells has ranged between 0.0% and 3.4% [34,37]. In our study, we predominantly used fresh or non-cryopreserved autologous grafts particularly for the first autologous HSCTs even after acquiring cryopreservation facilities. The median times for neutrophil engraftment and platelet engraftment after autologous HSCT were 11 and 17 days respectively. Additionally, TRM at day 100 post-autologous HSCT for all our MM patients who received their autologous transplants at inpatient or outpatient settings was 0.00%.
Studies have shown that HSCT without cryopreservation has the following advantages: allowing autologous HSCT to be performed entirely as outpatient due to the simplicity of its implementation, decreasing transplantation costs and the time between the last induction therapy and HD chemotherapy, prevention of toxicity of DMSO, no significant loss of viability of stem cells provided an infusion of the collected stem cells is made within 5 days of apheresis, expansion of the number of medical institutions performing HSCT and autologous graft versus host disease (GVHD) as well as potent ES [3,31,34-39]. However, HSCT without cryopreservation has the following disadvantages: plenty of coordination is needed between various teams regarding the timing of stem cell mobilization, apheresis, administration of conditioning therapy and infusion of stem cells; limitation of the use of standard HD chemotherapy schedules such as BEAM (BCNU, etoposide, cytarabine and melphalan) employed in the autologous HSCT for patients with lymphoma and inability to store part of the collection and reserving it for a second autologous HSCT in case a rich apheresis product is obtained [3,31,34,37].
In the 1990s and in an era where conventional chemotherapy was the only available treatment, the concept of up-front treatment with a tandem autologous HSCT was attempted to improve PFS and OS [40,41]. Updated results of the EMN02/HO95 trial concluded that double frontline autologous HSCT was superior to single autologous HSCT in terms of PFS and OS in all patients, particularly those having poor prognosis or HR subgroups of patients [42,43]. Tandem autologous HSCT has also been shown to overcome the expected poor outcome in patients with newly diagnosed MM having HR cytogenetics and extramedullary disease [44]. As compared with a single autologous HSCT, tandem transplantation improves OS among patients with myeloma, especially those who do not have a VGPR after undergoing the first transplantation [45]. In our study, HR-MM patients were planned for tandem autologous HSCTs but only patients who show favorable responses to the first autologous HSCTs were offered tandem transplants. Consequently, twelve of our HR-MM patients received tandem autografts.
Patients with MM are ideal candidates for outpatient autologous HSCT due to the ease of administration of HD melphalan, the relatively low extra-hematological toxicity, and the brief period of neutropenia [46-48]. There are specific inclusion criteria for outpatient HSCT and these include: (1) availability of full-time caregiver; (2) residence within 30 minutes drive from the hospital; (3) favorable comorbidity profile and performance status; (4) stable psychology and expected compliance; and (5) patient preference as well as a signed written consent [47,49-53]. On the other hand, the following criteria exclude patients from outpatient HSCT: (1) age more than 65 years; (2) performance status >1; (3) advanced comorbid medical conditions and severe impairment of organ functions; (4) severe recent infection or colonization with multidrug-resistant micro-organisms; (5) lack of caregiver as well as living > 1-hour drive distance from the hospital; and (6) advanced MM [49,54-56]. Occasionally, recipients of outpatient autologous need hospital admission for ≥1 of the following indications: (1) FN, pneumonia, sepsis, or arrhythmia; (2) severe mucositis and poor oral intake; and (3) declining performance status of the patient to the extent that the family or the caregiver become unable to cope [54,56-61]. In our study, 54 patients (40.00%) received their autologous transplants in an outpatient setting thus saving beds and reducing transplantation costs. We applied the inclusion and exclusion criteria outlined above for considering patients to have autologous HSCT at outpatient. Less than 30% of recipients of outpatient autologous HSCTs required admission for complications such as FN, sepsis, severe mucositis and ES. However, the median time for hospitalization post-HSCT was 3 days.
During the neutrophilic recovery following HSCT, a constellation of clinical manifestations referred to as ES may occur and these include: fever, erythematous skin rash, nausea, vomiting, diarrhea and noncardiogenic pulmonary edema [38,62]. Early recognition of ES is vital in order to administer appropriate GVHD therapy which includes HD corticosteroids, alemtuzumab, infliximab, daclizumab, and etanercept [38,62-66]. The incidence of ES in our study patients was 24%. Nevertheless, all patients who developed ES including the 2 complicated cases were managed successfully.
In patients with MM, maintenance therapy after autologous HSCT has been shown to deepen and prolong responses and increase OS and PFS [67,68]. The use of lenalidomide maintenance treatment after autologous HSCT in patients with MM had been investigated in 4 phase III randomized control studies which demonstrated a benefit in PFS [69-71]. Lenalidomide maintenance given after autologous HSCT till disease progression had become the standard of care in patients with newly diagnosed MM as it has been shown to prolong OS, PFS and event-free survival [68,70,72-75]. Bortezomib alone or in combination with other drugs such as dexamethasone, thalidomide, and pomalidomide has been shown to be safe, well tolerated, and efficacious in maintenance therapy following autologous HSCT particularly in patients with: HR cytogenetics including deletion 17p; renal insufficiency; previous history of another cancer; and inability to tolerate lenalidomide therapy [75-78]. Continuous therapy has become a key strategy in patients with MM as it has been shown to prolong the duration of remission and significantly improve OS and PFS [79-82]. Currently, continuous therapy till disease progression represents the standard approach for patients with MM both at diagnosis and at relapse as it provides better disease control [83]. Risk-adapted therapy is recommended as patients having HR-MM may benefit from more intensive maintenance treatment than patients with SR-MM [81]. Maintenance therapy was given to 75.6% of patients. Continuous therapy was administered to 52.9% of our MM patients. Surprisingly, the OS of patients with MM who received their autologous grafts between 2018 and 2022 and who received continuous therapy was significantly lower than that for patients who received their autografts between 2010 and 2017 and who received maintenance therapy for 1 to 2 years.
Despite including a relatively large number of patients in our study that extended over more than 11 years, we acknowledge that retrospective studies have their own limitations. Also, we are eager to know whether the findings reported in Figures 5 and 6 are reproduced by other studies.
Our patients developed MM at a much younger age than in western countries. Additionally, significant proportions of our patients presented with HR features such as advanced RISS stage, adverse cytogenetics and advanced bone disease. Autologous HSCT without cryopreservation is safe and feasible and leads not only to excellent short-term results but also to long-term outcomes that are at least comparable to the standard autologous transplantation with cryopreservation. Even after having cryopreservation facilities installed at our institution about 10 years ago, the excellent outcomes encountered encouraged us to continue offering fresh cells for the first autografts given to patients with MM. Fresh autologous grafts allowed us to perform 40% of autologous transplant procedures in outpatient settings in order to save beds and reduce costs. MM patients having severe renal dysfunction and even ESRD should not be excluded from autologous HSCT as they can benefit from autologous HSCT provided enough attention is given to the drug dose adjustment and timing of administering drugs in relation to hemodialysis.
Authors’ contributions
All authors participated in the management of the patients included in the study. Also, all authors read and approved the final form of the manuscript.
The authors are grateful to all medical, nursing and technical staff at KFSH in Dammam, Saudi Arabia who participated in the management of the patients included in this retrospective study.
- Małecki B, Gil L, Dytfeld D. Role of transplantation in treatment of multiple myeloma in era of novel agents. Acta Haematol Pol. 2021; 52(2): 77-84. doi: 10.5603/AHP.2021.0013.
- Ozaki S, Shimizu K. Autologous stem cell transplantation in elderly patients with multiple myeloma: past, present, and future. Biomed Res Int. 2014;2014:394792. doi: 10.1155/2014/394792. Epub 2014 Feb 20. PMID: 24719860; PMCID: PMC3956410.
- Al-Anazi KA. Autologous Hematopoietic Stem Cell Transplantation for Multiple Myeloma without Cryopreservation. Bone Marrow Res. 2012;2012:917361. doi: 10.1155/2012/917361. Epub 2012 May 28. PMID: 22693672; PMCID: PMC3368160.
- Du J, Zhuang J. Major advances in the treatment of multiple myeloma in American Society of Hematology annual meeting 2020. Chronic Dis Transl Med. 2021 Aug 31;7(4):220-226. doi: 10.1016/j.cdtm.2021.08.003. PMID: 34786541; PMCID: PMC8579022.
- Charliński G, Jurczyszyn A. Multiple myeloma - 2020 update on diagnosis and management. NOWOTWORY J Oncol 2020; 70: 85-91.
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch Arztebl Int. 2016 Jul 11;113(27-28):470-6. doi: 10.3238/arztebl.2016.0470. PMID: 27476706; PMCID: PMC4973001.
- Al-Anazi K. Hematopoietic stem cell transplantation in multiple myeloma in the era of novel therapies. In: Update on Multiple Myeloma. Edited by Khalid Al-Anazi. London. Intech Open. 2018. doi: 10.5772/intechopen.79999
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022 Aug;97(8):1086-1107. doi: 10.1002/ajh.26590. Epub 2022 May 23. PMID: 35560063; PMCID: PMC9387011.
- Padala SA, Barsouk A, Barsouk A, Rawla P, Vakiti A, Kolhe R, Kota V, Ajebo GH. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel). 2021 Jan 20;9(1):3. doi: 10.3390/medsci9010003. PMID: 33498356; PMCID: PMC7838784.
- Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016 Dec;43(6):676-681. doi: 10.1053/j.seminoncol.2016.11.004. Epub 2016 Nov 10. PMID: 28061985; PMCID: PMC5283695.
- Joshua DE, Bryant C, Dix C, Gibson J, Ho J. Biology and therapy of multiple myeloma. Med J Aust. 2019 May;210(8):375-380. doi: 10.5694/mja2.50129. Epub 2019 Apr 23. PMID: 31012120.
- Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019 Apr 8;9(4):44. doi: 10.1038/s41408-019-0205-9. PMID: 30962422; PMCID: PMC6453900.
- Bazarbachi AH, Al Hamed R, Malard F, Bazarbachi A, Harousseau JL, Mohty M. Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: an update. Blood Cancer J. 2022 Mar 28;12(3):47. doi: 10.1038/s41408-022-00645-1. PMID: 35347107; PMCID: PMC8960754.
- Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020 Sep 28;10(9):94. doi: 10.1038/s41408-020-00359-2. PMID: 32989217; PMCID: PMC7523011.
- Parrondo RD, Ailawadhi S, Sher T, Chanan-Khan AA, Roy V. Autologous Stem-Cell Transplantation for Multiple Myeloma in the Era of Novel Therapies. JCO Oncol Pract. 2020 Feb;16(2):56-66. doi: 10.1200/JOP.19.00335. PMID: 32045556.
- Drozd-Sokołowska J, Gras L, Zinger N, Snowden JA, Arat M, Basak G, Pouli A, Crawley C, Wilson KMO, Tilly H, Byrne J, Bulabois CE, Passweg J, Ozkurt ZN, Schroyens W, Lioure B, Colorado Araujo M, Poiré X, Van Gorkom G, Gurman G, de Wreede LC, Hayden PJ, Beksac M, Schönland SO, Yakoub-Agha I. Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation-study on behalf of CMWP of the EBMT. Bone Marrow Transplant. 2022 Apr;57(4):633-640. doi: 10.1038/s41409-022-01592-y. Epub 2022 Feb 15. PMID: 35169284; PMCID: PMC8993690.
- Rajkumar SV. Clinical features, laboratory manifestations, and diagnosis of multiple myeloma. Up to Date 2018. Edited by Kyle RA, Connor RF.
- Ricciuti G, Falcone A, Cascavilla N, Martinelli G, Cerchione C. Autologous stem cell transplantation in multiple myeloma. Panminerva Med. 2020 Dec;62(4):220-224. doi: 10.23736/S0031-0808.20.04114-2. Epub 2020 Sep 21. PMID: 32955179.
- Nishimura KK, Barlogie B, van Rhee F, Zangari M, Walker BA, Rosenthal A, Schinke C, Thanendrarajan S, Davies FE, Hoering A, Morgan GJ. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood Adv. 2020 Jan 28;4(2):422-431. doi: 10.1182/bloodadvances.2019000524. PMID: 31990333; PMCID: PMC6988393.
- Piriyakhuntorn P, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, Norasetthada L. Outcomes of Non-Cryopreserved Versus Cryopreserved Peripheral Blood Stem Cells for Autologous Stem Cell Transplantation in Multiple Myeloma. Ann Transplant. 2020 Dec 11;25:e927084. doi: 10.12659/AOT.927084. PMID: 33303730; PMCID: PMC7737409.
- Srour SA, Milton DR, Bashir Q, Nieto Y, Saini N, Daher M, Ramdial J, Im J, Hosing C, Delgado R, Manasanch E, Lee HC, Thomas S, Kaufman G, Patel K, Popat U, Weber D, Orlowski R, Shpall E, Champlin RE, Qazilbash MH. Melphalan dose intensity for autologous stem cell transplantation in multiple myeloma. Haematologica. 2021 Dec 1;106(12):3211-3214. doi: 10.3324/haematol.2021.279179. PMID: 34407606; PMCID: PMC8634193.
- Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, Dispenzieri A, Fonseca R, Sher T, Kyle RA, Lin Y, Russell SJ, Kumar S, Bergsagel PL, Zeldenrust SR, Leung N, Drake MT, Kapoor P, Ansell SM, Witzig TE, Lust JA, Dalton RJ, Gertz MA, Stewart AK, Rajkumar SV, Chanan-Khan A, Lacy MQ; Mayo Clinic. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013 Apr;88(4):360-76. doi: 10.1016/j.mayocp.2013.01.019. Erratum in: Mayo Clin Proc. 2013 Jul;88(7):777. Stewart, Keith [corrected to Stewart, A Keith]. PMID: 23541011.
- Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, Vesole DH, Jagannath S, Meyers R, Barlogie B. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996 Jun;2(6):947-52. PMID: 9816255.
- Parikh GC, Amjad AI, Saliba RM, Kazmi SM, Khan ZU, Lahoti A, Hosing C, Mendoza F, Qureshi SR, Weber DM, Wang M, Popat U, Alousi AM, Champlin RE, Giralt SA, Qazilbash MH. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009 Jul;15(7):812-6. doi: 10.1016/j.bbmt.2009.03.021. PMID: 19539212; PMCID: PMC4112358.
- El Fakih R, Fox P, Popat U, Nieto Y, Shah N, Parmar S, Oran B, Ciurea S, Kebriaei P, Hosing C, Ahmed S, Shah J, Orlowski R, Champlin R, Qazilbash M, Bashir Q. Autologous Hematopoietic Stem Cell Transplantation in Dialysis-Dependent Myeloma Patients. Clin Lymphoma Myeloma Leuk. 2015 Aug;15(8):472-6. doi: 10.1016/j.clml.2015.03.003. Epub 2015 Mar 27. PMID: 25963284; PMCID: PMC4516682.
- Mahindra A, Hari P, Fraser R, Fei M, Huang J, Berdeja J, Callander N, Costa L, Diaz MA, Freytes C, Gale RP, Girnius S, Holmberg L, Kharfan-Dabaja M, Kumar S, Kyle R, Lazarus H, Lee C, Maiolino A, Moreb J, Nishihori T, Pawarode A, Saad A, Savani BN, Schriber J, William B, Wirk BM, Krishnan A, Nieto Y, D'Souza A. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone Marrow Transplant. 2017 Dec;52(12):1616-1622. doi: 10.1038/bmt.2017.198. Epub 2017 Sep 18. PMID: 28920949; PMCID: PMC5859934.
- Al-Anazi KA, Mokhtar N, Kawari M, AlHashmi H, Abduljalil O, Alshaibani E, et al. A young patient with refractory multiple myeloma and dialysis-dependent renal failure has been cured by noncryopreserved autologous stem cell transplantation followed by liverelated kidney transplantation. J Stem Cell Bio. Transplant. 2017; 1 (2:13). doi: 10.21767/ 2575-7725.100013
- Katragadda L, McCullough LM, Dai Y, Hsu J, Byrne M, Hiemenz J, May S, Cogle CR, Norkin M, Brown RA, Wingard JR, Chang M, Moreb JS. Effect of melphalan 140 mg/m(2) vs 200 mg/m(2) on toxicities and outcomes in multiple myeloma patients undergoing single autologous stem cell transplantation-a single center experience. Clin Transplant. 2016 Aug;30(8):894-900. doi: 10.1111/ctr.12762. Epub 2016 Jun 29. PMID: 27219740.
- Knudsen LM, Nielsen B, Gimsing P, Geisler C. Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. Eur J Haematol. 2005 Jul;75(1):27-33. doi: 10.1111/j.1600-0609.2005.00446.x. PMID: 15946307.
- Antlanger M, Dust T, Reiter T, Böhm A, Lamm WW, Gornicec M, Willenbacher E, Nachbaur D, Weger R, Rabitsch W, Rasoul-Rockenschaub S, Worel N, Lechner D, Greinix H, Keil F, Gisslinger H, Agis H, Krauth MT. Impact of renal impairment on outcomes after autologous stem cell transplantation in multiple myeloma: a multi-center, retrospective cohort study. BMC Cancer. 2018 Oct 20;18(1):1008. doi: 10.1186/s12885-018-4926-0. PMID: 30342509; PMCID: PMC6195957.
- Wannesson L, Panzarella T, Mikhael J, Keating A. Feasibility and safety of autotransplants with noncryopreserved marrow or peripheral blood stem cells: a systematic review. Ann Oncol. 2007 Apr;18(4):623-32. doi: 10.1093/annonc/mdm069. Epub 2007 Mar 12. PMID: 17355952.
- Hechler G, Weide R, Heymanns J, Köppler H, Havemann K. Storage of noncryopreserved periphered blood stem cells for transplantation. Ann Hematol. 1996 May;72(5):303-6. doi: 10.1007/s002770050176. PMID: 8645742.
- Sarmiento M, Ramírez P, Parody R, Salas MQ, Beffermann N, Jara V, Bertín P, Pizarro I, Lorca C, Rivera E, Galleguillos M, Ocqueteau M, Sánchez-Ortega I, Patiño B, Sureda A. Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy. Bone Marrow Transplant. 2018 Aug;53(8):960-966. doi: 10.1038/s41409-018-0117-5. Epub 2018 Feb 13. PMID: 29440738.
- Ramzi M, Zakerinia M, Nourani H, Dehghani M, Vojdani R, Haghighinejad H. Non-cryopreserved hematopoietic stem cell transplantation in multiple myeloma, a single center experience. Clin Transplant. 2012 Jan-Feb;26(1):117-22. doi: 10.1111/j.1399-0012.2011.01432.x. Epub 2011 Sep 15. PMID: 21919958.
- Kayal S, Sharma A, Iqbal S, Tejomurtula T, Cyriac SL, Raina V. High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: a single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells. Clin Lymphoma Myeloma Leuk. 2014 Apr;14(2):140-7. doi: 10.1016/j.clml.2013.09.001. Epub 2013 Sep 28. PMID: 24342104.
- Bekadja MA, Brahimi M, Osmani S, Arabi A, Bouhass R, Yafour N, Entasoltan B, Rasheed W, Attaf F. A simplified method for autologous stem cell transplantation in multiple myeloma. Hematol Oncol Stem Cell Ther. 2012;5(1):49-53. doi: 10.5144/1658-3876.2012.49. PMID: 22446610.
- Jasuja SK, Kukar (jasuja) N, Jain R, Bhateja A, Jasuja A, Rohit Jain. A simplified method at lowest cost for autologous, non-cryopreserved, unmanipulated, peripheral hematopoietic stem cell transplant in multiple myeloma and non-Hodgkin's lymphoma: Asian scenario. J Clin Oncol. 2010; 28(15): ė18545
- Mutahar E, Al-Anazi KA. Engraftment syndrome: An updated review. J Stem Cell Transplant. 2017; 1(3):16. doi: 10.21767/2575-7725.100016
- Kanfar S, Al-Anazi KA. Autologous graft versus host disease: An updated review. Ann Stem Cells Regen Med. 2018; 1(1): 1002
- Barlogie B, Jagannath S, Vesole DH, Naucke S, Cheson B, Mattox S, Bracy D, Salmon S, Jacobson J, Crowley J, Tricot G. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997 Feb 1;89(3):789-93. PMID: 9028309.
- Putkonen M, Rauhala A, Itälä M, Kauppila M, Pelliniemi TT, Remes K. Double versus single autotransplantation in multiple myeloma; a single center experience of 100 patients. Haematologica. 2005 Apr;90(4):562-3. PMID: 15820960.
- Cavo M, Petrucci MT, Di Raimondo F, Zamagni E, Gamberi B, Crippa C, et al. Upfront single versus double autologous stem cell transplantation for newly diagnosed multiple myeloma: an intergroup, multicenter, phase III study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood. 2016; 128, 991. doi:10.1182/blood. V128.22.991.991
- Cavo M, Gay FM, Patriarca F, Zamagni E, Montefusco V, Dozza L, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/H095 study. Blood 2017; 130:401. doi: 10.1182/blood.V130. Suppl_1.401.401
- Gagelmann N, Eikema DJ, Koster L, Caillot D, Pioltelli P, Lleonart JB, Reményi P, Blaise D, Schaap N, Trneny M, Passweg J, Porras RP, Cahn JY, Musso M, Poiré X, Fenk R, Itälä-Remes M, Pavone V, Fouillard L, Maertens J, Bron D, Pouli A, Schroyens W, Schönland S, Garderet L, Yakoub-Agha I, Kröger N. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019 Nov;25(11):2134-2142. doi: 10.1016/j.bbmt.2019.07.004. Epub 2019 Jul 6. PMID: 31288095.
- Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R; InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003 Dec 25;349(26):2495-502. doi: 10.1056/NEJMoa032290. Erratum in: N Engl J Med. 2004 Jun17;350(25):2628. PMID: 14695409.
- Martino M, Lemoli RM, Girmenia C, Castagna L, Bruno B, Cavallo F, Offidani M, Scortechini I, Montanari M, Milone G, Postacchini L, Olivieri A. Italian consensus conference for the outpatient autologous stem cell transplantation management in multiple myeloma. Bone Marrow Transplant. 2016 Aug;51(8):1032-40. doi: 10.1038/bmt.2016.79. Epub 2016 Apr 4. PMID: 27042841.
- Martino M, Montanari M, Bruno B, Console G, Irrera G, Messina G, Offidani M, Scortechini I, Moscato T, Fedele R, Milone G, Castagna L, Olivieri A. Autologous hematopoietic progenitor cell transplantation for multiple myeloma through an outpatient program. Expert Opin Biol Ther. 2012 Nov;12(11):1449-62. doi: 10.1517/14712598.2012.707185. Epub 2012 Jul 13. PMID: 22788745.
- Dytfeld D, Łojko-Dankowska A, Nowicki A, Matuszak M, Wache A, Gil L. Safety and cost effectiveness of outpatient autologous hematopoietic stem cell transplantation for multiple myeloma - singlecenter experience of a pilot Early Discharge Program. Acta Haematol Pol. 2021; 52(3): 178-181. doi: 10.5603/ AHP.a2021.0029·
- Graff TM, Singavi AK, Schmidt W, Eastwood D, Drobyski WR, Horowitz M, Palmer J, Pasquini M, Rizzo DJ, Saber W, Hari P, Fenske TS. Safety of outpatient autologous hematopoietic cell transplantation for multiple myeloma and lymphoma. Bone Marrow Transplant. 2015 Jul;50(7):947-53. doi: 10.1038/bmt.2015.46. Epub 2015 Apr 13. PMID: 25867651; PMCID: PMC4490016.
- Martino M, Paviglianiti A, Memoli M, Martinelli G, Cerchione C. Multiple Myeloma Outpatient Transplant Program in the Era of Novel Agents: State-of-the-Art. Front Oncol. 2020 Nov 11;10:592487. doi: 10.3389/fonc.2020.592487. PMID: 33262948; PMCID: PMC7686536.
- Khouri J, Majhail NS. Advances in delivery of ambulatory autologous stem cell transplantation for multiple myeloma. Curr Opin Support Palliat Care. 2017 Dec;11(4):361-365. doi: 10.1097/SPC.0000000000000305. PMID: 28922292.
- Paul TM, Liu SV, Chong EA, Luger SM, Porter DL, Schuster SJ, Tsai DE, Nasta SD, Loren A, Frey N, Perl A, Cohen AD, Weiss BM, Stadtmauer EA, Vogl DT. Outpatient Autologous Stem Cell Transplantation for Patients With Myeloma. Clin Lymphoma Myeloma Leuk. 2015 Sep;15(9):536-40. doi: 10.1016/j.clml.2015.05.006. Epub 2015 Jun 6. PMID: 26141214.
- Abid MB, Christopher D, Abid MA, Poon ML, Tan LK, Koh LP, Chng WJ. Safety and cost-effectiveness of outpatient autologous transplantation for multiple myeloma in Asia: single-center perspective from Singapore. Bone Marrow Transplant. 2017 Jul;52(7):1044-1046. doi: 10.1038/bmt.2017.77. Epub 2017 May 8. PMID: 28481354.
- Lisenko K, Sauer S, Bruckner T, Egerer G, Goldschmidt H, Hillengass J, Schmier JW, Shah S, Witzens-Harig M, Ho AD, Wuchter P. High-dose chemotherapy and autologous stem cell transplantation of patients with multiple myeloma in an outpatient setting. BMC Cancer. 2017 Feb 22;17(1):151. doi: 10.1186/s12885-017-3137-4. PMID: 28228122; PMCID: PMC5322605.
- Frey P, Stinson T, Siston A, Knight SJ, Ferdman E, Traynor A, O'Gara K, Rademaker A, Bennett C, Winter JN. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant. 2002 Dec;30(11):741-8. doi: 10.1038/sj.bmt.1703676. PMID: 12439696.
- Larsen K, Spencer H, Mohan M, Bailey C, Hill K, Kottarathara M, Parikh R, Hoque S, Erra A, Mitma AA, Mathur P, Yarlagadda L, Gundarlapalli S, Ogunsesan Y, Hussain M, Thalambedu N, Sehti J, Al Hadidi S, Thanendrarajan S, Graziutti M, Zangari M, Barlogie B, van Rhee F, Tricot G, Schinke C. Feasibility of Outpatient Stem Cell Transplantation in Multiple Myeloma and Risk Factors Predictive of Hospital Admission. J Clin Med. 2022 Mar 16;11(6):1640. doi: 10.3390/jcm11061640. PMID: 35329966; PMCID: PMC8955129.
- Jagannath S, Vesole DH, Zhang M, Desikan KR, Copeland N, Jagannath M, Bracy D, Jones R, Crowley J, Tricot G, Barlogie B. Feasibility and cost-effectiveness of outpatient autotransplants in multiple myeloma. Bone Marrow Transplant. 1997 Sep;20(6):445-50. doi: 10.1038/sj.bmt.1700900. PMID: 9313876.
- Gertz MA, Ansell SM, Dingli D, Dispenzieri A, Buadi FK, Elliott MA, Gastineau DA, Hayman SR, Hogan WJ, Inwards DJ, Johnston PB, Kumar S, Lacy MQ, Leung N, Micallef IN, Porrata LF, Schafer BA, Wolf RC, Litzow MR. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008 Oct;83(10):1131-8. doi: 10.4065/83.10.1131. PMID: 18828972.
- Martino M, Russo L, Martinello T, Gallo GA, Fedele R, Moscato T, Console G, Vincelli DI, Ronco F, Postorino M, Irrera G, Messina G. A home-care, early discharge model after autografting in multiple myeloma: results of a three-arm prospective, non-randomized study. Leuk Lymphoma. 2015 Mar;56(3):801-4. doi: 10.3109/10428194.2014.931952. Epub 2014 Jul 17. PMID: 24913501.
- Ferrara F, Izzo T, Criscuolo C, Riccardi C, Viola A, Delia R, Carbone A, Celentano M. Comparison of fixed dose pegfilgrastim and daily filgrastim after autologous stem cell transplantation in patients with multiple myeloma autografted on a outpatient basis. Hematol Oncol. 2011 Sep;29(3):139-43. doi: 10.1002/hon.978. Epub 2010 Nov 30. PMID: 21922508.
- Faucher C, Le Corroller Soriano AG, Esterni B, Vey N, Stoppa AM, Chabannon C, Mohty M, Michallet M, Bay JO, Genre D, Maraninchi D, Viens P, Moatti JP, Blaise D. Randomized study of early hospital discharge following autologous blood SCT: medical outcomes and hospital costs. Bone Marrow Transplant. 2012 Apr;47(4):549-55. doi: 10.1038/bmt.2011.126. Epub 2011 Jul 4. PMID: 21725375.
- Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001 May;27(9):893-8. doi: 10.1038/sj.bmt.1703015. PMID: 11436099.
- Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Mod Pathol. 2011 Jan;24(1):117-25. doi: 10.1038/modpathol.2010.163. Epub 2010 Oct 15. PMID: 20953169.
- Nellen RG, van Marion AM, Frank J, Poblete-Gutiérrez P, Steijlen PM. Eruption of lymphocyte recovery or autologous graft-versus-host disease? Int J Dermatol. 2008 Nov;47 Suppl 1:32-4. doi: 10.1111/j.1365-4632.2008.03956.x. PMID: 18986483.
- Drobyski WR, Hari P, Keever-Taylor C, Komorowski R, Grossman W. Severe autologous GVHD after hematopoietic progenitor cell transplantation for multiple myeloma. Bone Marrow Transplant. 2009 Jan;43(2):169-77. doi: 10.1038/bmt.2008.295. Epub 2008 Sep 1. PMID: 18762759.
- Porrata LF. Clinical evidence of autologous graft versus tumor effect. Am J Immunol. 2009; 5(1): 1-7. doi: 10.3844/ajisp.2009.1.7
- Vaxman I, Gertz M. Risk adapted post-transplant maintenance in multiple myeloma. Expert Rev Hematol. 2019 Feb;12(2):107-118. doi: 10.1080/17474086.2019.1576521. PMID: 30696304.
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L, Decaux O, Leyvraz S, Vekemans MC, Voillat L, Michallet M, Pegourie B, Dumontet C, Roussel M, Leleu X, Mathiot C, Payen C, Avet-Loiseau H, Harousseau JL; IFM Investigators. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1782-91. doi: 10.1056/NEJMoa1114138. PMID: 22571202.
- Manasanch EE. Recommend maintenance therapy with lenalidomide in multiple myeloma. Semin Oncol. 2016 Dec;43(6):712-713. doi: 10.1053/j.seminoncol.2016.11.002. Epub 2016 Nov 5. PMID: 28061994.
- Goldschmidt H, Mai EK, Dürig J, Scheid C, Weisel KC, Kunz C, Bertsch U, Hielscher T, Merz M, Munder M, Lindemann HW, Hügle-Dörr B, Tichy D, Giesen N, Hose D, Seckinger A, Huhn S, Luntz S, Jauch A, Elmaagacli A, Rabold B, Fuhrmann S, Brossart P, Goerner M, Bernhard H, Hoffmann M, Hillengass J, Raab MS, Blau IW, Hänel M, Salwender HJ; German-speaking Myeloma Multicenter Group (GMMG). Response-adapted lenalidomide maintenance in newly diagnosed myeloma: results from the phase III GMMG-MM5 trial. Leukemia. 2020 Jul;34(7):1853-1865. doi: 10.1038/s41375-020-0724-1. Epub 2020 Feb 7. PMID: 32034285.
- Holstein SA, Suman VJ, Hillengass J, McCarthy PL. Future Directions in Maintenance Therapy in Multiple Myeloma. J Clin Med. 2021 May 24;10(11):2261. doi: 10.3390/jcm10112261. PMID: 34073689; PMCID: PMC8197068.
- McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, Bringhen S, Musto P, Anderson KC, Caillot D, Gay F, Moreau P, Marit G, Jung SH, Yu Z, Winograd B, Knight RD, Palumbo A, Attal M. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017 Oct 10;35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679. Epub 2017 Jul 25. PMID: 28742454; PMCID: PMC5652871.
- Syed YY. Lenalidomide: A Review in Newly Diagnosed Multiple Myeloma as Maintenance Therapy After ASCT. Drugs. 2017 Sep;77(13):1473-1480. doi: 10.1007/s40265-017-0795-0. PMID: 28791622.
- Uyl-de Groot CA, Ramsden R, Lee D, Boersma J, Zweegman S, Dhanasiri S. Lenalidomide as maintenance treatment for patients with multiple myeloma after autologous stem cell transplantation: A pharmaco-economic assessment. Eur J Haematol. 2020 Nov;105(5):635-645. doi: 10.1111/ejh.13497. Epub 2020 Sep 12. PMID: 32705720; PMCID: PMC7590122.
- Baertsch MA, Mai EK, Hielscher T, Bertsch U, Salwender HJ, Munder M, Fuhrmann S, Dührsen U, Brossart P, Neben K, Schlenzka J, Kunz C, Raab MS, Hillengaß J, Jauch A, Seckinger A, Hose D, Luntz S, Sonneveld P, Lokhorst H, Martin H, Goerner M, Hoffmann M, Lindemann HW, Bernhard H, Blau IW, Scheid C, Besemer B, Weisel KC, Hänel M, Dürig J, Goldschmidt H; German-Speaking Myeloma Multicenter Group (GMMG). Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2021 Jan 7;11(1):1. doi: 10.1038/s41408-020-00390-3. PMID: 33414374; PMCID: PMC7791127.
- Sivaraj D, Green MM, Li Z, Sung AD, Sarantopoulos S, Kang Y, Long GD, Horwitz ME, Lopez RD, Sullivan KM, Rizzieri DA, Chao NJ, Gasparetto C. Outcomes of Maintenance Therapy with Bortezomib after Autologous Stem Cell Transplantation for Patients with Multiple Myeloma. Biol Blood Marrow Transplant. 2017 Feb;23(2):262-268. doi: 10.1016/j.bbmt.2016.11.010. Epub 2016 Nov 14. PMID: 27856369.
- Bird SA, Jackson GH, Pawlyn C. Maintenance Strategies Post-Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma. Clin Hematol Int. 2020 May 20;2(2):59-68. doi: 10.2991/chi.d.200502.001. PMID: 34595444; PMCID: PMC8432350.
- Sahebi F, Frankel PH, Farol L, Krishnan AY, Cai JL, Somlo G, Thomas SH, Reburiano E, Popplewell LL, Parker PM, Spielberger RT, Kogut NM, Karanes C, Htut M, Ruel C, Duarte L, Murata-Collins JL, Forman SJ. Sequential bortezomib, dexamethasone, and thalidomide maintenance therapy after single autologous peripheral stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2012 Mar;18(3):486-92. doi: 10.1016/j.bbmt.2011.12.580. Epub 2011 Dec 22. PMID: 22198542.
- Dimopoulos MA, Jakubowiak AJ, McCarthy PL, Orlowski RZ, Attal M, Bladé J, Goldschmidt H, Weisel KC, Ramasamy K, Zweegman S, Spencer A, Huang JSY, Lu J, Sunami K, Iida S, Chng WJ, Holstein SA, Rocci A, Skacel T, Labotka R, Palumbo A, Anderson KC. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020 Feb 13;10(2):17. doi: 10.1038/s41408-020-0273-x. PMID: 32054831; PMCID: PMC7018731.
- Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, Nagler A, Petrucci MT, Hajek R, Pezzatti S, Delforge M, Patriarca F, Donato F, Cerrato C, Nozzoli C, Yu Z, Boccadifuoco L, Caravita T, Benevolo G, Guglielmelli T, Vincelli D, Jacques C, Dimopoulos MA, Ciccone G, Musto P, Corradini P, Cavo M, Boccadoro M. Continuous Therapy Versus Fixed Duration of Therapy in Patients With Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2015 Oct 20;33(30):3459-66. doi: 10.1200/JCO.2014.60.2466. Epub 2015 Aug 17. PMID: 26282661.
- D'Agostino M, De Paoli L, Conticello C, Offidani M, Ria R, Petrucci MT, Spada S, Marcatti M, Catalano L, Gilestro M, Guglielmelli T, Baldini L, Gamberi B, Rizzi R, De Sabbata G, Di Renzo N, Patriarca F, Pezzatti S, Siniscalchi A, Ribolla R, Palumbo A, Montefusco V, Nagler A, Boccadoro M, Gay F. Continuous therapy in standard- and high-risk newly-diagnosed multiple myeloma: A pooled analysis of 2 phase III trials. Crit Rev Oncol Hematol. 2018 Dec;132:9-16. doi: 10.1016/j.critrevonc.2018.09.008. Epub 2018 Sep 14. PMID: 30447931.
- Ozaki S, Handa H, Koiso H, Saitoh T, Sunami K, Ishida T, Suzuki K, Narita T, Iida S, Nakamura Y, Suzuki K, Nishimura N, Murakami H, Shimizu K. Propensity-score matched analysis of the efficacy of maintenance/continuous therapy in newly diagnosed patients with multiple myeloma: a multicenter retrospective collaborative study of the Japanese Society of Myeloma. J Cancer Res Clin Oncol. 2022 Jan;148(1):191-203. doi: 10.1007/s00432-021-03668-6. Epub 2021 Jun 2. PMID: 34080068.
- Bonello F, Cetani G, Bertamini L, Gay F, Larocca A. Moving Toward Continuous Therapy in Multiple Myeloma. Clin Hematol Int. 2019 Nov 12;1(4):189-200. doi: 10.2991/chi.d.191101.001. PMID: 34595430; PMCID: PMC8432368.