More Information
Submitted: February 28, 2023 | Approved: March 06, 2023 | Published: March 08, 2023
How to cite this article: Al-Anazi KA, Alshami A, Mutahar E, Abduljalil O, Kanfer S, et al. Outcome of Outpatient Autologous Hematopoietic Stem Cell Transplantation in Patients with Multiple Myeloma and Relapsed and Refractory Hodgkin Lymphoma. The Experience of King Fahad Specialist Hospital in Dammam, Saudi Arabia. J Stem Cell Ther Transplant. 2023; 7: 003-015.
DOI: 10.29328/journal.jsctt.1001030
Copyright License: © 2023 Al-Anazi KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Multiple myeloma; Hodgkin lymphoma; Autologous hematopoietic stem cell transplantation; Non-cryopreservation; Outpatient transplantation
Outcome of Outpatient Autologous Hematopoietic Stem Cell Transplantation in Patients with Multiple Myeloma and Relapsed and Refractory Hodgkin Lymphoma. The Experience of King Fahad Specialist Hospital in Dammam, Saudi Arabia
Khalid Ahmed Al-Anazi1*, Alshami A1, Mutahar E1, Abduljalil O1, Kanfer S1, Kaloyannidis P1, Bacal J1, Estanislao A1, Apostolidis I1, Almokhtar N1, Darweesh M1, Abdulbaqi M1, Alenazi W1, Alshammasi Z1, Albanyan O1, Ayyad A1, Alsomali Z1, Albatran M1, Raslan H2, Albahrani A3, Alsaber A2, AlMulhem N3, Dridi W4, Alrabeh R2, Abu Rahma F1, Nightingale F1, Ahadai P1 and Alhashmi H1
1Department of Hematology and Hematopoietic Stem Cell Transplantation, Oncology Center, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
2Hematopathology and Flowcytometry, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
3Apheresis and Blood Bank, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
4Cytogenetics, Department of Pathology and Central Laboratory, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia
*Address for Correspondence: Dr. Khalid Ahmed Al-Anazi, Consultant Hemato-Oncologist, Department of Hematology and Hematopoietic Stem Cell Transplantation, Oncology Center, King Fahad Specialist Hospital, P.O. Box: 15215, Dammam 31444, Saudi Arabia, Email: [email protected]
Background: Autologous hematopoietic stem cell transplants (HSCT) is the standard of care for transplant-eligible patients with newly diagnosed multiple myeloma (MM) and patients with relapsed and refractory Hodgkin lymphoma (R/R-HL) who achieve chemosensitivity after salvage therapy. Although autologous HSCT is routinely performed in an inpatient setting, the procedure can safely be performed in an outpatient setting.
Methods and materials: A retrospective study of patients with MM and R/R- HL who received outpatient autologous HSCT at King Fahad Specialist Hospital (KFSH) in Dammam, Saudi Arabia between the first of April 2017 and the 31st of January 2022 was performed.
Results: Over the study period of 4 years and 10 months, a total of 90 outpatient autologous HSCTs were performed for 79 patients (54 patients with MM; 4 of them received planned tandem autografts and 7 other myeloma patients received second autologous HSCTs for relapsed or progressive disease; and 25 patients with R/R-HL) at our institution. The median ages of patients with MM and those with R/R-HL at HSCT were 50.4 years and 27.8 years respectively.
At the presentation of their MM, the following high-risk (HR) features were encountered: stage II and III diseases according to the revised international scoring system (RISS) in 53.7%; adverse cytogenetics in 42.6% and extensive bone involvement in 53.7% of patients. In patients with HL at presentation, 48% of patients had stage IV disease according to Ann Arbor staging classification and 84% of patients had B symptoms.
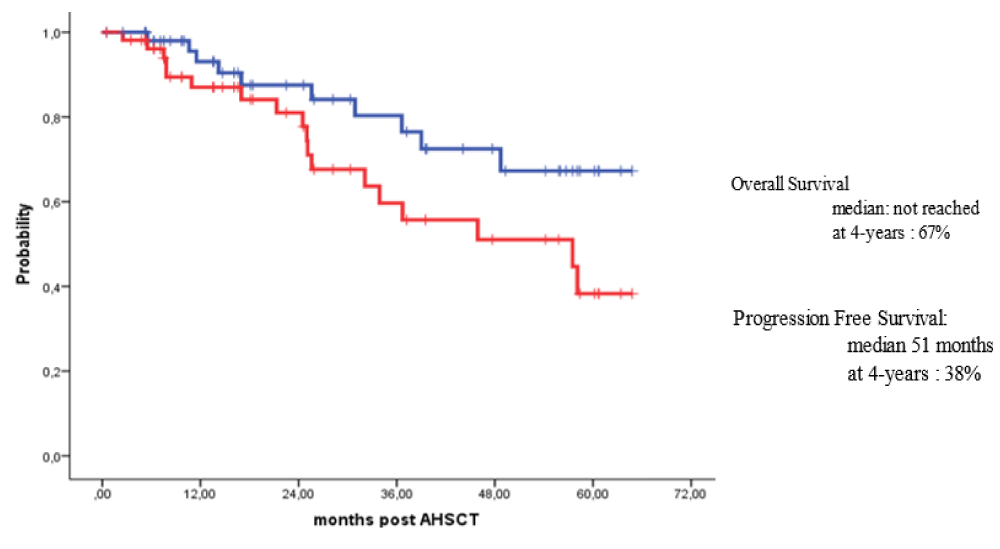
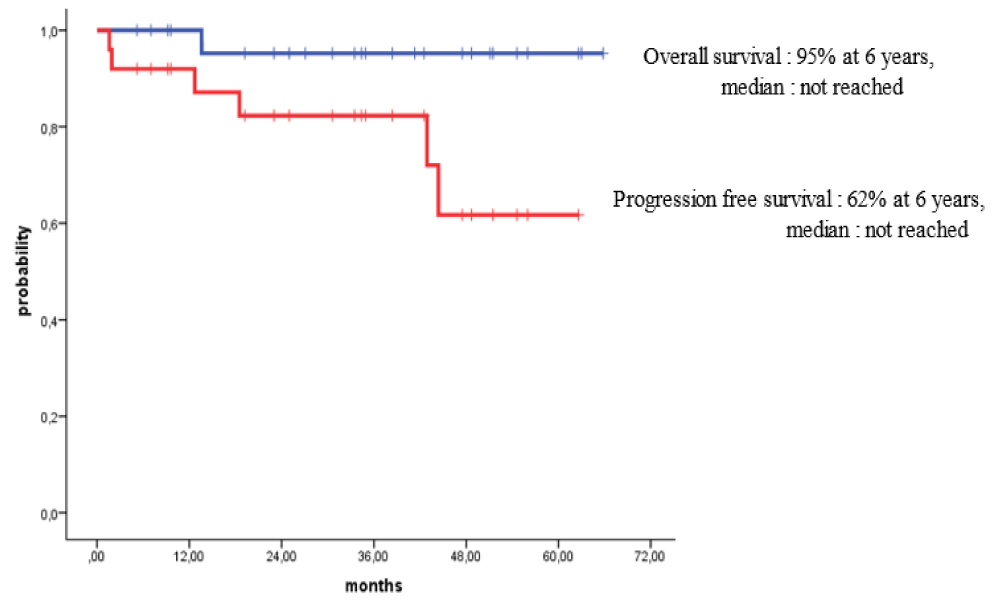
Survival for 100 days post-HSCT for all patients with MM and HL who received outpatient autologous transplants was 100%. For patients with MM, the overall survival (OS) rates at 3 years and 4 years post-HSCT were 80% and 67%, while the progression-free survival (PFS) rates over 3 years and 4 years were 58% and 38% respectively. For patients with HL, the OS at 6 years post-HSCT was 95% while the PFS rates at 3 years and 6 years post-HSCT were 84% and 62% respectively.
Conclusion: Outpatient autologous HSCT for patients with MM and HL is safe, and feasible and can lead to short-term as well as long-term outcomes that are comparable to autologous transplantation performed in an inpatient setting. Additional benefits of outpatient autologous include saving beds and reducing hospital costs.
MM is characterized by the proliferation of monoclonal plasma cells in the bone marrow and the production of monoclonal proteins as well as the occurrence of secondary end-organ damage [1-7]. The recent utilization of various novel therapies such as proteasome inhibitors, immunomodulatory agents and monoclonal antibodies in the treatment of patients with newly diagnosed MM and relapsed disease has improved not only the depth and duration of disease response but also the OS [8,9]. In patients with newly diagnosed MM, VRd (bortezomib, lenalidomide and dexamethasone) regimen is recommended as the standard first-line treatment while in patients with HR-MM, the addition of daratumumab has been shown to improve the efficacy and prolong survival [4,7,10-13]. However, despite the use of several lines of novel therapies, MM has remained an incurable disease [14,15]. Hence, there is a need to: (1) develop novel targeting therapies with different mechanisms of action to achieve deep and durable responses in an attempt to cure MM; and (2) identify tumor intrinsic and extrinsic resistance mechanisms in order to direct the design of combinations of novel drugs that can prevent or overcome drug resistance to improve patient survival [14,15].
HL is an uncommon B-cell lymphoid malignancy that accounts for 10% - 15% of all lymphomas [16-18]. Although it mostly affects young individuals, HL has a second peak of incidence in patients > 60 years of age [18]. Approximately 80% of patients with HL can be cured with initial chemotherapy and radiotherapy. However, 10% -30% of patients relapse and 5% - 10% of patients develop refractory disease [16,19-22]. Initial treatment of HL is based on: the histology of the disease, anatomical stage, and presence or absence of poor prognostic features [17]. The incorporation of functional imaging in the management of HL allows adjustment or modification of treatment and identifies patients who may benefit from additional therapeutic interventions such as radiotherapy [23]. Currently, positron emission tomography (PET)-adapted chemotherapy and radiotherapy approaches are utilized in the initial treatment of early-stage HL and have resulted in OS and PFS of 95% and 85% respectively [24,25]. Novel agents including the antibody-drug conjugate brentuximab vedotin (BV) and the checkpoint inhibitors such as nivolumab and pembrolizumab can improve the effectiveness of HL treatment and have been shown to extend OS in patients with R/R-HL [16,22,23,26,27]. However, in patients with advanced disease, early incorporation of novel therapies even in the frontline regimens may improve the outcome of these patients who carry poor prognoses [24,28,29].
In patients with R/R-HL, several regimens of chemotherapy and novel agents have been employed as salvage therapy including (1) dexamethasone + BEAM (BCNU, etoposide, cytarabine, melphalan) or mini-MEAM that includes all the chemotherapeutic agents included in BEAM regimen but with dose reductions; (2) ICE (ifosfamide, carboplatin, etoposide); (3) DHAP (dexamethasone, cytarabine, cisplatin); (4) ESHAP (methylprednisolone, cisplatin, etoposide, cytarabine); (5) MINE (mitoguazone, ifosfamide, vinorelbine, etoposide); (6) GDP (gemcitabine, dexamethasone, cisplatin) or GVD (gemcitabine, dexamethasone, liposomal doxorubicine); (7) IV (ifosfamide, vinorelbine) or IEV (ifosfamide, etoposide, vinorelbine); and (8) BV-based therapies such as BV + checkpoint inhibitors; BV + bendamustine (BVB); BV + ICE; and BV + dexamethasone + HD cytarabine + cisplatin [30-41]. However, there is no obvious superior salvage regimen although maintaining dose intensity is important for optimal responses [42]. Nevertheless, the use of a DICEP regimen (dose-intensive cyclophosphamide + etoposide + cisplatin) has been shown to be feasible, well tolerated, and effective with favorable long-term outcomes when used as salvage as well as stem cell mobilization regimen prior to high-dose (HD) chemotherapy and autologous HSCT in patients with lymphoma [43-45]. In patients with R/R HL, a DICEP regimen of chemotherapy can lead to excellent long-term outcomes that may be superior to the less intensive salvage regimens [43-45]. GDP regimen which is used as salvage therapy in patients with R/R HL has a high response rate, favorable toxicity profile, and excellent mobilization potential. Additionally, it can safely be administered in an outpatient setting [46].
A retrospective study was conducted between the 1st of April 2017 and the 31st of January 2022. The medical records, the clinical data as well as the laboratory data of all patients with MM and HL who received autologous HSCT at KFSH in Dammam, Saudi Arabia during the time specified above were retrieved for analysis. For our cryopreserved autologous HSCTs, after controlling the primary disease using certain induction therapeutic regimens, mobilization of stem cells was performed using cyclophosphamide, ESHAP, DHAP, DICEP, or filgrastim alone, then the collection of mobilized stem cells by apheresis was done followed by cryopreservation of the apheresis product. After the administration of HD melphalan or other conditioning therapies, the cryopreserved stem cells were infused after thawing. However, for non-cryopreserved autologous grafts, the same process was followed with the exception of keeping the collected stem cells at 4 ºC for 24 to 72 hours instead of cryopreserving them. Then the fresh stem cells were infused within 24 hours after administration of HD melphalan or other conditioning regimens.
During stem cell mobilization, once the CD34+ cell count in peripheral blood exceeded 10.0 to 20.0 × 106/kg body weight, stem cell collection by leukapheresis was usually commenced. We aimed to obtain a target of 3.0 to 4.0 × 106 CD34+ cells/kg in case a single auto-HSCT was desired and a target of 6.0 to 8.0 × 106 CD34+ cells/kg in case a tandem transplant for MM was planned. After day 0 of autologous HSCT, prophylactic antimicrobials were administered, and starting from day 5 post-HSCT till the day of neutrophil engraftment daily doses of filgrastim were administered.
Statistical analysis
The SSPS version 22 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The Kaplan-Meier method with a log-rank test was used to estimate the survival rates and to identify risk factors that influenced the treatment outcome. OS was defined as the duration from the day of graft infusion until death or the date of the last follow-up for live patients. PFS was defined as the period from graft infusion till the documentation of disease relapse/progression or last follow-up for the non-relapsed/progressed patients.
During the study period, 4 years and 10 months, a total of 90 outpatient autologous HSCTs were performed for 79 patients (54 with MM and 25 patients with HL) at KFSH in Dammam, Saudi Arabia. Single autologous grafts were offered to all 25 patients with HL and 54 patients with MM. Four MM patients received planned tandem autologous HSCTs while 7 other myeloma patients received second autologous grafts due to relapse or progression of their MM after receiving appropriate salvage therapies. Out of the 79 recipients of outpatient autologous HSCT, there were 41 males and 38 females and the median age of patients at HSCT was 50.4 years for MM patients and 27.8 years for patients with HL.
In patients with MM at the presentation of their disease, the following HR features were encountered: stage II and III diseases according to the RISS in 53.7% of patients, adverse cytogenetics in 42.6% and extensive bone involvement in 53.7% of patients, while 11.20% of patients had either renal dysfunction or end-stage renal disease (ESRD) (Tables 1-4). Out of the 65 outpatient autologous HSCTs, 39 autologous HSCTs (60.0%) were performed using non-cryopreserved stem cells while 26 autologous grafts (40.0%) used cryopreserved stem cells. Regarding the initial therapy administered to MM patients, 46 patients (85.2%) of our patients received bortezomib-based therapy either the doublet regimen bortezomib and dexamethasone (VD) or one of the triplet regimens [VRd; bortezomib, cyclophosphamide, dexamethasone (VCD); or bortezomib, thalidomide, dexamethasone (VTD)] and only 6 patients (11.1%) received more intensive regimens containing PACE chemotherapy (cisplatin, doxorubicin, cyclophosphamide, and etoposide) including VTD-PACE, VRd-PACE and carfilzomib, lenalidomide, dexamethasone (KRd)-PACE (Table 5). The number of lines of chemotherapy administered prior to autologous HSCT was as follows: 40 patients (74.1%) received 1 line of therapy, 8 patients (14.8%) received 2 lines of therapy, while 6 patients (11.1%) received ≥ 3 lines of chemotherapy. Treatment responses in MM patients before outpatient autologous HSCT were as follows: 4 patients (7.4%) achieved partial response (PR), 33 patients (61.1%) achieved very good PR (VGPR) and 16 patients (29.6%) achieved complete response (CR) while only 1 patient (1.9%) achieved stringent CR. Regarding the maintenance therapy administered to patients with MM after outpatient autologous HSCT, 6 patients (11.1%) received maintenance therapy for 1 to 2 years and 44 patients (81.5%) received maintenance treatment till disease progression, while 4 patients (7.4%) did not receive maintenance therapy.
| Table 1: Stage of disease in patients with MM subjected to outpatient auto-HSCT. | ||
| Stage | Number | Percentage |
| I | 23 | 42.6 |
| II | 19 | 35.2 |
| III | 10 | 18.5 |
| Unknown | 2 | 3.7 |
| • MM: multiple myeloma; • auto-HSCT: autologous hematopoietic stem cell transplantation | ||
| Table 2: Cytogenetic abnormalities in patients with MM subjected to outpatient auto-HSCT. | ||
| Cytogenetic abnormality | Number | Percentage |
| Normal | 18 | 33.3 |
| Chromosome 3 abnormalities including deletion of chromosome 3 |
5 | 9.25 |
| 17 p Deletion | 5 | 9.25 |
| Translocation of chromosome 14 including: t 4,14; t 6,14; t 14,16; t 14,20 |
9 | 16.7 |
| Trisomies of chromosomes: 3,7,9,15,17 |
7 | 13.0 |
| Monosomies of chromosomes: 13; 16 |
4 | 7.4 |
| Not available; Unknown | 6 | 11.1 |
| •MM: multiple myeloma •auto-HSCT: autologous hematopoietic stem cell transplantation | ||
| Table 3: Bone lesions in patients with MM subjected to outpatient auto-HSCT. | ||
| Type of Bone Involvement | Number | Percentage |
| Localized or single lytic lesion (s) | 8 | 14.8 |
| Multiple lytic lesions | 24 | 44.4 |
| Pathological fractures requiring surgery | 5 | 9.3 |
| Osteopenia | 17 | 31.5 |
| •MM: multiple myeloma •auto-HSCT: autologous hematopoietic stem cell transplantation | ||
| Table 4: Renal dysfunction in patients with MM subjected to outpatient auto-HSCT. | ||
| Type of renal dysfunction | Number | Percentage |
| End stage renal disease (ESRD) on hemodialysis [serum creatinine: 629 -1328 μmol/L] Creatinine clearance < 10 mL/minute |
2 | 3.7 |
| ESRD not yet on hemodialysis [serum creatinine: 381- 477 μmol/L] Creatinine clearance: 10-20 mL/minute |
1 | 1.9 |
| Significant renal dysfunction [serum creatinine: 185 - 204 μmol/L] Creatinine clearance: 20-30 mL/minute |
3 | 5.6 |
| • MM: multiple myeloma • auto-HSCT: autologous hematopoietic stem cell transplantation | ||
| Table 5: Initial therapy given to patients with MM subjected to outpatient auto-HSCT. | ||
| Regimen/Protocol | Number of patients | Percentage |
| Bortezomib + Dexamethasone [VD] | 10 | 18.5 |
| Bortezomib triplet protocols VTD/VCD/VRd |
36 | 66.7 |
| Lenalidomide + dexamethasone [RD] | 2 | 3.7 |
| More intensive regimens VTD-PACE / VRd-PACE / KRd-PACE |
6 | 11.1 |
| MM: Multiple Myeloma; Auto-HSCT: autologous Hematopoietic Stem Cell Trans-plantation; VCd: bortezomib, cyclophosphamide, dexamethasone; VTd: bortezomib, thalidomide, dexamethasone; VRd: bortezomib, lenalidomide, dexamethasone; VTD-PACE: bortezomib, thalidomide, dexamethasone, cisplatin, adriamycin, cyclo-phosphamide, etoposide; KRd: carfilzomib, lenalidomide, dexamethasone. | ||
Out of the 25 patients with HL subjected to outpatient autologous HSCT, there were 14 males and 11 females and their ages ranged between 16 and 52 years with a median age of 27.8 years at the time of HSCT (Table 6). Regarding the histological subtypes: 12 patients (48%) had nodular sclerosis classical HL, while 6 patients (24%) had mixed cellularity subtypes. Additionally, 12 patients (48%) had stage IV disease at presentation, 21 patients (84%) had B symptoms, and 4 patients (16%) had the bulky disease and another 4 patients (16%) had the disease at extranodal sites (Table 6). Several salvage regimens of chemotherapy were given to HL patients at the relapse or progression of their disease. Seventeen patients (68%) required a single line of salvage therapy while 8 patients (32%) had 2-5 lines of salvage treatment. Two to four cycles of BVB were given to 10 patients (40%), while 1 cycle of ESHAP chemotherapy was given to 2 patients (8%) and 1 cycle of DICEP was given to 5 patients (20%) (Table 7).
| Table 6: Pre-treatment characteristics of patients with HL subjected to outpatient autologous HSCT. | |
| Characteristic | Details |
| Age | Range: 16-52 |
| Gender | Males: 14; Females: 11 |
| Classical HL subtype | Nodular sclerosis: 12 (48%) Mixed cellularity: 6 (24%) Unclassified: 7 (28%) |
| Disease stage at diagnosis | II: 7 (28%); III: 6 (24%); IV: 12 (48%) |
| B symptoms | 21 (84%) |
| Bulky disease | 4 (16%) |
| Extranodal sites | 4 (16%) |
| •HL: Hodgkin Lymphoma; •HSCT: Hematopoietic Stem Cell Transplantation | |
| Table 7: Salvage therapy given for disease progression or relapse in patients with HL subjected to outpatient auto-HSCT. | |||
| Complication | Specific regimens | Number | Percentage |
| Single line of salvage therapy | ESHAP (1 cycle) | 2 | 8 |
| DICEP (1 cycle) | 5 | 20 | |
| BVB (2-4 cycles) | 10 | 40 | |
| Combined therapy [2-5 lines of various combinations of the following regimens: ESHAP; DHAP; DICEP; BVB; NIVO; IGEV] |
2 lines | 6 | 24 |
| 3 lines | 1 | 4 | |
| 4 lines | 1 | 4 | |
| •HL: Hodgkin Lymphoma; •auto-HSCT: autologous Hematopoietic Stem Cell Trans- plantation; •ESHAP: Methylprednisolone, Cisplatin, Etoposide, Cytarabine; •DHAP: Dexamethasone, Cytarabine, Cisplatin; •DICEP: Dose-IntensiveCyclophosphamide, Etoposide, Cisplatin; •BVB: Brentuximab Vedotin; •IGEV: Ifosfamide, Gemcitabine and Vinorelbine; • NIVO: Nivolumab |
|||
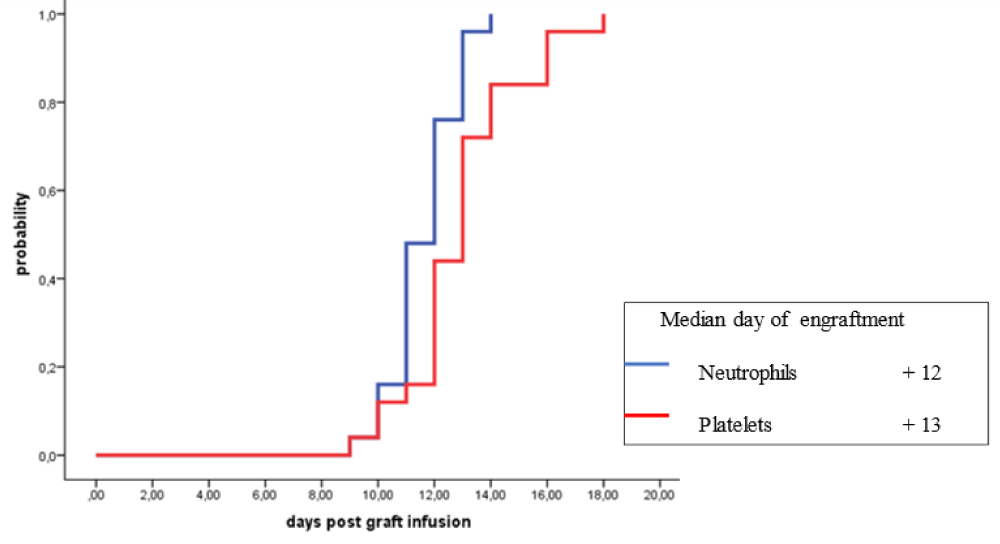
Five different stem cell mobilization regimens were used in patients with HL subjected to outpatient autologous HSCT and these included: cyclophosphamide in 9 patients (36%), DICEP in 8 patients (32%), ESHAP in 4 patients (16%), DHAP in 2 patients (8%) and granulocyte-colony stimulating factor (G-CSF) alone in 2 patients (8%) (Table 8). The following early post-HSCT complications were encountered in HL patients: febrile neutropenia (FN) in 8 patients (32%), infectious complications in 4 patients (16%) and mucositis in 2 patients (8%) (Table 9). The median days of engraftment for neutrophils and platelets for patients with R/R-HL were 12 days and 13 days post-HSCT respectively (Figure 1).
| Table 8: Stem cell mobilization regimens used in patients with HL subjected to outpatient auto-HSCT. | ||
| Regimen | Number | Percentage |
| DICEP | 8 | 32 |
| Cyclophosphamide | 9 | 36 |
| ESHAP | 4 | 16 |
| DHAP | 2 | 8 |
| G-CSF alone | 2 | 8 |
| •HL: Hodgkin Lymphoma; •auto-HSCT: autologous Hematopoietic Stem Cell Trans- plantation; •DICEP: Dose-Intensive Cyclophosphamide, Etoposide, Cisplatin; •ESHAP: Methylprednisolone, Cisplatin, Etoposide, Cytarabine; •DHAP: Dexame-thasone, Cytarabine, Cisplatin; •G-CSF: Granulocyte Colony Stimulating Factor |
||
| Table 9: Post-transplant complications encountered in patients with HL subjected to outpatient auto-HSCT. | ||
| Complication | Number | Percentage |
| Febrile neutropenia | 8 | 32 |
| Mucositis | 2 | 8 |
| Infectious complications | 4 | 16 |
| No complications | 2 | 48 |
| •HL: Hodgkin Lymphoma; •auto-HSCT: autologous Hematopoietic Stem Cell Trans-plantation | ||
Figure 1: Engraftment of neutrophils and platelets in patients with relapsed/refractory Hodgkin lymphoma autografted with single agent high dose melphalan
Survival at day 100 post-HSCT for all patients with MM and HL who received their outpatient autologous HSCTs was 100%. Also, the long-term outcomes of our patients were excellent. For patients with MM subjected to outpatient autologous HSCT, the OS rates at 3 years and 4 years post-HSCT were 80% and 67%, while PFS rates over 3 years and 4 years were 58% and 38% respectively (Figure 2). For patients with HL subjected to outpatient autologous HSCT, the OS at 6 years post-HSCT was 95% while PFS rates at 3 years and 6 years post-HSCT were 84% and 62% respectively (Figure 3).
Figure 2: Overall and survival free survival for patient with Multiple myeloma autografted in outpatient basis.
Figure 3: Fibrohyalinized
Autologous HSCT is a widely accepted therapeutic strategy for the treatment of certain hematologic malignancies (HMs) and it is most frequently indicated for patients with MM and lymphoma [9,47]. However, eligibility for autologous HSCT is determined by several factors including age, performance status, presence and severity of comorbid medical conditions, and frailty score as frailty has been shown to be a predictor of short survival and is considered an exclusion criterion for autologous HSCT [7,8,48,49]. Cryopreservation using the cryopreservative dimethyl sulfoxide is routinely employed after stem cell collection prior to autologous HSCT [3,7,50]. However, several old and recent studies in addition to one systematic review have shown that autologous HSCT using non-cryopreserved stem cells is safe, cost-effective and leads to short-term as well as long-term results that are at least equivalent to autologous HSCT using cryopreserved stem cells [7,47,50-55]. One of the advantages of autologous HSCT without cryopreservation is the simplicity of its implementation which allows the performance of autologous HSCT as an outpatient [3,7,56]. In our 65 outpatient autologous HSCTs performed for patients with MM, non-cryopreserved stem cells were used in 39 autografts (60.0%), while cryopreserved stem cells were given to the other 40% of HSCTs, particularly in the tandem or second autologous grafts.
Despite the availability of several lines of novel agents, autologous HSCT is still considered the standard of care in the treatment of patients with MM who are eligible for transplantation [2,7,8,13,57]. The standard conditioning regimen for patients with MM undergoing autologous HSCT is HD melphalan (200 mg/m2) given intravenously (IV) [5,7,8,13,58]. However, in patients with renal dysfunction or failure, dose reductions to 100 mg/m2 - 140 mg/m2 are needed according to creatinine clearance [5,7]. In our study, HD melphalan was the conditioning therapy given to our MM patients. However, melphalan dose adjustments were offered to patients having creatinine clearance < 20 mL/minute at the time of autologous HSCT. After stem cell mobilization with cyclophosphamide, G-CSF and plerixafor which is used in case of poor mobilization, peripheral blood stem cells are collected using an apheresis machine aiming to collect at least 2.5 x 106/kilogram body weight to guarantee successful autologous graft [3,13,47].
HD chemotherapy and autologous HSCT can salvage 40% - 70% of patients with R/R HL [59]. Hence, salvage therapy followed by HD chemotherapy and autologous HSCT has become the standard of care for most patients with primary refractory disease or those who relapse after initial therapy [16,17,19-21,60]. In patients with R/R HL, studies have shown that HD chemotherapy followed by autologous HSCT can lead to 5 years of OS of 55% to 63% and 5 years of PFS and event-free survival (EFS) of 44% and 51.3% respectively [61,62]. However, the use of BV and checkpoint inhibitors after autologous HSCT in patients with R/R HL has further improved the long-term outcomes with 5 years of PFS ranging between 59% and 73.4% and 5 years OS reaching 92% [25,63,64].
In patients with lymphoma, BEAM conditioning therapy has traditionally been administered over 6 days as an inpatient [60,65]. Studies have shown that the BEAM regimen can safely be administered in an outpatient setting in order to: reduce costs and length of hospitalization, decrease risks of severe toxicities and infectious complications, and improve patient satisfaction and quality of life [60,65]. In patients with HL, several other chemotherapeutic regimens have been employed in the conditioning therapy prior to autologous HSCT as alternatives to BEAM chemotherapy and these include: (1) a mini-BEAM regimen, (2) the addition of rituximab or anti-CD 25 radioimmunotherapy to BEAM regimen, (3) modification of BEAM regimen such as the use of TEAM regimen (thiotepa, etoposide, cytarabine, melphalan) and (4) replacing BEAM chemotherapy by other regimens such as BEC (BCNU, etoposide, cyclophosphamide); etoposide and melphalan; mitoxantrone and melphalan; as well as busulfan, cyclophosphamide, and etoposide [66-77]. Studies have shown that the use of HD melphalan alone given as conditioning therapy prior to autologous HSCT in patients with advanced or R/R HL has the following advantages: (1) being adequate and cost-effective with outcomes that are comparable to multiagent chemotherapeutic regimens such as BEAM, (2) less exposure to other toxic chemotherapies, and (3) allowing autologous HSCT to be performed in an outpatient setting [78-81]. In our patients with R/R-HL, HD melphalan proved to be an adequate conditioning therapy and it allowed outpatient autologous HSCT to be performed.
In patients with R/R HL, particularly those who relapse after autologous HSCT but have a chemo-sensitive disease, allogeneic HSCT may be the only potentially curative modality of treatment that can offer long-term survival [21,82]. HD BEAM administered as an inpatient for 6 consecutive days has traditionally been used as conditioning therapy given prior to autologous HSCT in patients with lymphoma [60,65]. However, outpatient administration of the BEAM regimen has been shown to be safe and can offer several advantages including reductions in the length of hospitalization and costs [60].
While historically, due to logistic issues and concerns regarding toxicities and infections, most of the autologous HSCTs were performed in an inpatient setting, the swift recovery after peripheral autologous HSCT and improvements in supportive care have enabled patients to receive autologous HSCT at outpatient [83,84]. It has been reported that outpatient autologous HSCT is safe and feasible in patients with lymphoma, central nervous system tumors, and breast cancer [83,85-87]. Allogeneic HSCT with reduced intensity conditioning therapy as well as haploidentical allogeneic HSCT have been performed in an outpatient setting in the following diseases: MM; R/R lymphoma; Sezary syndrome; and other R/R HMs [88-94]. Even total body irradiation has been given successfully in an outpatient setting [94].
Due to the ease of administration of HD melphalan, the relatively low extra-hematological toxicity, and the short period of neutropenia, patients with MM are ideal candidates for outpatient autologous HSCT [95-97]. Additionally, several studies have shown that autologous HSCT can safely be performed in an outpatient setting in patients with HL and non-Hodgkin lymphoma in order to save healthcare resources without compromising patient outcomes [65,85,98-100]. With daily outpatient clinical evaluation and intensive supportive care; outpatient autologous HSCT is safe, feasible, and cost-effective. Also, it can lead to excellent short-term and long-term outcomes in carefully selected patients with MM and lymphoma [83,97,101-114]. However, it is essential to have HSCT- specific supportive interventions that address the multidisciplinary and complex requirements of both patients and their caregivers by optimising the involvement of the key stakeholders throughout the entire process from stem cell mobilization to passing the first 100 days post-HSCT [115]. Therefore, a multidisciplinary approach with close follow-up is required to guarantee a successful outcome of the autologous outpatient HSCT program [105,106,113,116].
Several studies have clearly indicated that outpatient HSCT has certain inclusion criteria that include: (1) availability of full-time caregiver; (2) good performance status; (3) favorable comorbidity profile; (4) residence within 20 to 30 minutes drive from the hospital; (5) stable psychological status; (6) patient preference; (7) expected compliance; and (8) signed written consent [83,97,101,102,107,114]. On the other hand, the exclusion criteria of outpatient HSCT include: (1) age more than 65 years; (2) performance status > 1; (3) lack of caregiver; (4) > 1-hour drive distance between home and hospital; (5) advanced disease such as MM or lymphoma (6) advanced comorbid medical conditions; (7) severe impairment of organ functions; (8) serious infection either encountered recently or not completely eradicated; (9) colonization with multidrug-resistant bacteria or fungus; and (10) no guaranteed availability of quick readmission to the hospital once hospitalization is needed [84,96,109,117]. In our study, we followed the inclusion and exclusion criteria outlined above for selecting patients to be candidates for outpatient autologous HSCT for patients with MM and R/R- HL.
The risk factors that can predict admission in recipients of outpatient autologous HSCT include (1) advanced disease, (2) female gender, (3) poor performance status, (4) low serum albumin level, and (5) more intensive conditioning regimens such as BEAM chemotherapy [84]. Indications for admission in recipients of outpatient HSCT include (1) severe mucositis requiring narcotic analgesia or total parenteral nutrition (TPN); (2) FN; (3) poor oral intake or uncontrolled nausea, vomiting, or diarrhea requiring TPN or aggressive hydration; (4) inability of family or caregiver to cope; (5) declining performance status of the patient; and (6) presence of other serious complications such as pneumonia, sepsis or arrhythmia [84,106,109-112,118]. Between 8% and 84% of recipients of outpatient autologous HSCT require hospitalization in the first 100 days post-HSCT ranges [84,103,106,109-113,119]. Duration of hospitalization ranges between 4 and 9 days and the most frequent day of unexpected hospitalization is day 7 post-autologous HSCT [103,105,106,110,112]. In our patients, readmission rates after HSCT were higher for patients with R/R-HL than patients with MM. In both groups of patients, mucositis, FN and infectious complications were the main reasons for readmissions. Additionally, vacant hospital beds were made available to guarantee the safety of patients transplanted in an outpatient setting.
The reported median time to engraftment in patients with MM receiving autologous HSCT at outpatient is 9-14 days for neutrophils and 12-19 days for platelets [106,108-112]. In our patients with MM, the median time for neutrophil engraftment with G-CSF given from the 5th-day post-HSCT onwards was 11 days while the median time for platelet engraftment post autologous HSCT was 17 days. However, in patients with R/R-HL, the median days of engraftment for neutrophils with G-CSF were given from day 5 post-HSCT onwards and platelets were 12 days and 13 days post-HSCT respectively (Figure 1). The reported transplant-related mortality (TRM) in recipients of autologous transplantation performed in an outpatient is 0.0% - 1.1% [96,101,103,106,111-114,116]. In our study, TRM at day 100 post-HSCT was 0.0% for patients with MM and patients with R/R-HL.
Outpatient autologous HSCT has several advantages that include: (1) significant reduction in costs; (2) alleviation of constraints of chronic bed shortage; (3) significantly lower overall resource utilization; (4) patient convenience and high patient satisfaction; (5) lower rate of infectious complications; and (6) lower rates of morbidity as well as TRM [96,102,105,
109,113,116,120,121]. Reductions in treatment costs, saving hospital beds, and convenience as well as the satisfaction of patients were the main advantages of our outpatient autologous HSCT program.
In patients with MM, maintenance therapy after autologous HSCT has been shown to deepen and prolong responses and increase OS and PFS [122]. Lenalidomide maintenance given after autologous HSCT till disease progression had become the standard of care in patients with newly diagnosed MM as it has been shown to prolong PFS and EFS [123-126]. Bortezomib maintenance therapy after autologous HSCT in MM patients has been shown to be safe, well tolerated, and efficacious particularly in patients with: HR cytogenetics including deletion 17p, renal insufficiency, inability to tolerate lenalidomide, and those with a previous history of another cancer [127-129]. Compared to the traditional fixed-duration approaches, the evolving paradigm of continuous therapy and maintenance treatment offers prolonged disease control and improved outcomes in patients with MM. Currently, continuous therapy till disease progression represents the standard approach for patients with MM both at diagnosis and at relapse [130]. In our patients with MM, maintenance therapy with variable duration was given to 47 patients (87.04%).
The AETHERA trial (phase III randomized, placebo-controlled trial that included 325 patients with HL and was performed at 78 sites in USA and Europe between April 6, 2010 and September 21, 2012) showed that early consolidation with BV after autologous HSCT significantly improved PFS in patients at risk of relapse or progression following HSCT [131]. Subsequently, 2 other clinical trials performed in patients with HR or R/R HL showed that BV maintenance therapy following autologous HSCT improved the 2-year PFS between 67.75% and 75% [132,133]. Hence, it is recommended to intervene early by administering BV or other novel agents in patients with HL who are at risk of relapse or progression following autologous HSCT in order to improve their outcome [134-136]. In our patients with R/R-HL, maintenance therapy with a BV regimen was given to 5 patients (20%) while post-HSCT radiotherapy was offered to 7 patients (28%).
Our patients developed MM and HL at a much younger age than in western countries. Significant proportions of our patients presented with HR features such as advanced disease stage. Outpatient autologous HSCT has specific inclusion and exclusion criteria and requires daily clinical evaluation, and intensive supportive care including correction of electrolytic disturbances, and administration of needed blood products and antimicrobials.
Autologous HSCT performed in an outpatient setting is safe, and feasible and leads not only to excellent short-term results but also to long-term outcomes that are at least comparable to the standard inpatient autologous transplantation. Conditioning therapy with HD melphalan and the use of non-cryopreserved autologous stem cells allowed us to perform autologous transplantation in outpatient settings. Advantages of performing outpatient autologous HSCT include saving beds, reducing hospital costs, and lowering the rates of infections and TRM.
Authors’ contributions
All authors participated in the management of the patients included in the study. Also, all authors read and approved the final form of the manuscript.
The authors are grateful to all medical, nursing, and technical staff at KFSH in Dammam, Saudi Arabia who participated in the management of the patients included in this retrospective study.
- Ozaki S, Shimizu K. Autologous stem cell transplantation in elderly patients with multiple myeloma: past, present, and future. Biomed Res Int. 2014;2014:394792. doi: 10.1155/2014/394792. Epub 2014 Feb 20. PMID: 24719860; PMCID: PMC3956410.
- Małecki B, Gil L, Dytfeld D. Role of transplantation in treatment of multiple myeloma in era of novel agents. Acta Haematol Pol. 2021; 52(2): 77-84. doi: 10.5603/AHP.2021.0013.
- Al-Anazi KA. Autologous Hematopoietic Stem Cell Transplantation for Multiple Myeloma without Cryopreservation. Bone Marrow Res. 2012;2012:917361. doi: 10.1155/2012/917361. Epub 2012 May 28. PMID: 22693672; PMCID: PMC3368160.
- Du J, Zhuang J. Major advances in the treatment of multiple myeloma in American Society of Hematology annual meeting 2020. Chronic Dis Transl Med. 2021 Aug 31;7(4):220-226. doi: 10.1016/j.cdtm.2021.08.003. PMID: 34786541; PMCID: PMC8579022.
- Charliński G, Jurczyszyn A. Multiple myeloma - 2020 update on diagnosis and management. NOWOTWORY J Oncol. 2020; 70: 85-91.
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch Arztebl Int. 2016 Jul 11;113(27-28):470-6. doi: 10.3238/arztebl.2016.0470. PMID: 27476706; PMCID: PMC4973001.
- Al-Anazi K. Hematopoietic stem cell transplantation in multiple myeloma in the era of novel therapies. In: Update on Multiple Myeloma. Edited by Khalid Al-Anazi. London, Intech Open. 2018. doi: 10.5772/intechopen. 79999
- Parrondo RD, Ailawadhi S, Sher T, Chanan-Khan AA, Roy V. Autologous Stem-Cell Transplantation for Multiple Myeloma in the Era of Novel Therapies. JCO Oncol Pract. 2020 Feb;16(2):56-66. doi: 10.1200/JOP.19.00335. PMID: 32045556.
- Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli D, Dispenzieri A, Fonseca R, Hayman SR, Kapoor P, Kourelis TV, Lacy MQ, Larsen JT, Muchtar E, Reeder CB, Sher T, Stewart AK, Warsame R, Go RS, Kyle RA, Leung N, Lin Y, Lust JA, Russell SJ, Zeldenrust SR, Fonder AL, Hwa YL, Hobbs MA, Mayo AA, Hogan WJ, Rajkumar SV, Kumar SK, Gertz MA, Roy V. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant. 2019 Mar;54(3):353-367. doi: 10.1038/s41409-018-0264-8. Epub 2018 Jul 9. PMID: 29988062; PMCID: PMC6463224.
- Bazarbachi AH, Al Hamed R, Malard F, Bazarbachi A, Harousseau JL, Mohty M. Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: an update. Blood Cancer J. 2022 Mar 28;12(3):47. doi: 10.1038/s41408-022-00645-1. PMID: 35347107; PMCID: PMC8960754.
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020 May;95(5):548-567. doi: 10.1002/ajh.25791. Erratum in: Am J Hematol. 2020 Nov;95(11):1444. PMID: 32212178.
- Offidani M, Corvatta L, Morè S, Nappi D, Martinelli G, Olivieri A, Cerchione C. Daratumumab for the Management of Newly Diagnosed and Relapsed/Refractory Multiple Myeloma: Current and Emerging Treatments. Front Oncol. 2021 Feb 17;10:624661. doi: 10.3389/fonc.2020.624661. PMID: 33680948; PMCID: PMC7928404.
- Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019 Apr 8;9(4):44. doi: 10.1038/s41408-019-0205-9. PMID: 30962422; PMCID: PMC6453900.
- Nishida H. Rapid Progress in Immunotherapies for Multiple Myeloma: An Updated Comprehensive Review. Cancers (Basel). 2021 May 31;13(11):2712. doi: 10.3390/cancers13112712. PMID: 34072645; PMCID: PMC8198014.
- Swamydas M, Murphy EV, Ignatz-Hoover JJ, Malek E, Driscoll JJ. Deciphering mechanisms of immune escape to inform immunotherapeutic strategies in multiple myeloma. J Hematol Oncol. 2022 Feb 16;15(1):17. doi: 10.1186/s13045-022-01234-2. PMID: 35172851; PMCID: PMC8848665.
- Voorhees TJ, Beaven AW. Therapeutic Updates for Relapsed and Refractory Classical Hodgkin Lymphoma. Cancers (Basel). 2020 Oct 8;12(10):2887. doi: 10.3390/cancers12102887. PMID: 33050054; PMCID: PMC7601361.
- Ansell SM. Hodgkin lymphoma: A 2020 update on diagnosis, risk-stratification, and management. Am J Hematol. 2020 Aug;95(8):978-989. doi: 10.1002/ajh.25856. Epub 2020 Jun 8. PMID: 32384177.
- Momotow J, Borchmann S, Eichenauer DA, Engert A, Sasse S. Hodgkin Lymphoma-Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. J Clin Med. 2021 Mar 8;10(5):1125. doi: 10.3390/jcm10051125. PMID: 33800409; PMCID: PMC7962816.
- Rybka J, Wróbel T. Treatment of Hodgkin lymphoma relapse after autologous hematopoietic cell transplantation. Acta Haematol Pol. 2021; 52(4): 309-313. DOI: 10.5603/AHP.2021.0059
- Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, Arora M, Ramsay NK, Orchard PJ, MacMillan ML, Burns LJ. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006 Oct;12(10):1065-72. doi: 10.1016/j.bbmt.2006.06.006. PMID: 17084370.
- Iqbal N, Kumar L, Iqbal N. Update on salvage options in relapsed/refractory hodgkin lymphoma after autotransplant. ISRN Oncol. 2014 Mar 30;2014:605691. doi: 10.1155/2014/605691. PMID: 25006506; PMCID: PMC4003874.
- Al-Juhaishi T, Borogovac A, Ibrahimi S, Wieduwilt M, Ahmed S. Reappraising the Role of Allogeneic Hematopoietic Stem Cell Transplantation in Relapsed and Refractory Hodgkin's Lymphoma: Recent Advances and Outcomes. J Pers Med. 2022 Jan 18;12(2):125. doi: 10.3390/jpm12020125. PMID: 35207613; PMCID: PMC8880200.
- Spinner MA, Advani RH, Connors JM, Azzi J, Diefenbach C. New Treatment Algorithms in Hodgkin Lymphoma: Too Much or Too Little? Am Soc Clin Oncol Educ Book. 2018 May 23;38:626-636. doi: 10.1200/EDBK_200679. PMID: 30231319.
- Blum KA. Controversies in the management of early-stage Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):234-239. doi: 10.1182/hematology.2021000255. PMID: 34889367; PMCID: PMC8791127.
- Wali R, Saeed H, Patrus N, Javed S, Khan SJ. Outcomes of Refractory and Relapsed Hodgkin Lymphoma With Autologous Stem-Cell Transplantation: A Single Institution Experience. J Glob Oncol. 2019 Nov;5:1-6. doi: 10.1200/JGO.19.00051. PMID: 31756138; PMCID: PMC6882513.
- Ishizawa K, Yanai T. Hematopoietic Stem Cell Transplantation and Brentuximab Vedotin for Patients with Relapsed or Refractory Hodgkin Lymphoma and Systemic Anaplastic Large-Cell Lymphoma. Adv Ther. 2019 Oct;36(10):2679-2696. doi: 10.1007/s12325-019-01046-w. Epub 2019 Aug 7. PMID: 31392578; PMCID: PMC6822829.
- Merryman RW, Redd RA, Nishihori T, Chavez J, Nieto Y, Darrah JM, Rao U, Byrne MT, Bond DA, Maddocks KJ, Spinner MA, Advani RH, Ballard HJ, Svoboda J, Singh AK, McGuirk JP, Modi D, Ramchandren R, Romancik J, Cohen JB, Frigault MJ, Chen YB, Serritella AV, Kline J, Ansell S, Nathan S, Rahimian M, Joyce RM, Shah M, David KA, Park S, Beaven AW, Habib A, Bachanova V, Nakhoda S, Khan N, Lynch RC, Smith SD, Ho VT, LaCasce A, Armand P, Herrera AF. Autologous stem cell transplantation after anti-PD-1 therapy for multiply relapsed or refractory Hodgkin lymphoma. Blood Adv. 2021 Mar 23;5(6):1648-1659. doi: 10.1182/bloodadvances.2020003556. PMID: 33710337; PMCID: PMC7993097.
- Mohty R, Dulery R, Bazarbachi AH, Savani M, Hamed RA, Bazarbachi A, Mohty M. Latest advances in the management of classical Hodgkin lymphoma: the era of novel therapies. Blood Cancer J. 2021 Jul 9;11(7):126. doi: 10.1038/s41408-021-00518-z. PMID: 34244478; PMCID: PMC8270913.
- Tomassetti S, Herrera AF. Update on the role of brentuximab vedotin in classical Hodgkin lymphoma. Ther Adv Hematol. 2018 Jul 12;9(9):261-272. doi: 10.1177/2040620718786833. PMID: 30210755; PMCID: PMC6130098.
- Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011 Apr 21;117(16):4208-17. doi: 10.1182/blood-2010-09-288373. Epub 2011 Jan 24. PMID: 21263152.
- Fedele R, Martino M, Recchia AG, Irrera G, Gentile M, Morabito F. Clinical Options in Relapsed or Refractory Hodgkin Lymphoma: An Updated Review. J Immunol Res. 2015;2015:968212. doi: 10.1155/2015/968212. Epub 2015 Dec 16. PMID: 26788526; PMCID: PMC4695673.
- Takiar R, Karimi Y. Novel Salvage Therapy Options for Initial Treatment of Relapsed/Refractory Classical Hodgkin's Lymphoma: So Many Options, How to Choose? Cancers (Basel). 2022 Jul 20;14(14):3526. doi: 10.3390/cancers14143526. PMID: 35884585; PMCID: PMC9318183.
- Lynch RC, Cassaday RD, Smith SD, Fromm JR, Cowan AJ, Warren EH, Shadman MS, Shustov A, Till BG, Ujjani CS, Libby EN 3rd, Philip M, Coye H, Martino CN, Bhark SL, Morris K, Rasmussen H, Behnia S, Voutsinas J, Gopal AK. Dose-dense brentuximab vedotin plus ifosfamide, carboplatin, and etoposide for second-line treatment of relapsed or refractory classical Hodgkin lymphoma: a single centre, phase 1/2 study. Lancet Haematol. 2021 Aug;8(8):e562-e571. doi: 10.1016/S2352-3026(21)00170-8. PMID: 34329577; PMCID: PMC8457616.
- Pinczés LI, Szabó R, Illés Á, Földeák D, Piukovics K, Szomor Á, Gopcsa L, Miltényi Z. Real-world efficacy of brentuximab vedotin plus bendamustine as a bridge to autologous hematopoietic stem cell transplantation in primary refractory or relapsed classical Hodgkin lymphoma. Ann Hematol. 2020 Oct;99(10):2385-2392. doi: 10.1007/s00277-020-04204-1. Epub 2020 Aug 3. PMID: 32748163; PMCID: PMC7481161.
- LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M, Behler C, Cheung E, Forero-Torres A, Vose J, O'Connor OA, Josephson N, Wang Y, Advani R. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018 Jul 5;132(1):40-48. doi: 10.1182/blood-2017-11-815183. Epub 2018 Apr 27. PMID: 29703778; PMCID: PMC6073588.
- Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, Brice P, Hutchings M, Gastinne T, Liu R, Burggraaff CN, Nijland M, Tonino SH, Arens AIJ, Valkema R, van Tinteren H, Lopez-Yurda M, Diepstra A, De Jong D, Hagenbeek A. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2021 Apr 1;106(4):1129-1137. doi: 10.3324/haematol.2019.243238. PMID: 32273476; PMCID: PMC8018114.
- Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, Christian BA, Ansell SM, Moskowitz CH, Brown L, Zhang C, Taft D, Ansari S, Sacchi M, Ho L, Herrera AF. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021 Aug 12;138(6):427-438. doi: 10.1182/blood.2020009178. PMID: 33827139.
- Epperla N, Hamadani M. Double-refractory Hodgkin lymphoma: tackling relapse after brentuximab vedotin and checkpoint inhibitors. Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):247-253. doi: 10.1182/hematology.2021000256. PMID: 34889401; PMCID: PMC8791097.
- Moskowitz AJ, Herrera AF, Beaven AW. Relapsed and Refractory Classical Hodgkin Lymphoma: Keeping Pace With Novel Agents and New Options for Salvage Therapy. Am Soc Clin Oncol Educ Book. 2019 Jan;39:477-486. doi: 10.1200/EDBK_238799. Epub 2019 May 17. PMID: 31099645.
- Vassilakopoulos TP, Asimakopoulos JV, Konstantopoulos K, Angelopoulou MK. Optimizing outcomes in relapsed/refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies. Ther Adv Hematol. 2020 Feb 16;11:2040620720902911. doi: 10.1177/2040620720902911. PMID: 32110285; PMCID: PMC7026824.
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018 Mar;68(2):116-132. doi: 10.3322/caac.21438. Epub 2017 Dec 1. PMID: 29194581; PMCID: PMC5842098.
- Hertzberg M. Relapsed/refractory Hodgkin lymphoma: what is the best salvage therapy and do we need RIC-alloSCT? Hematol Oncol Clin North Am. 2014 Feb;28(1):123-47. doi: 10.1016/j.hoc.2013.09.001. PMID: 24287072.
- Stewart DA, Guo D, Glück S, Morris D, Chaudhry A, deMetz C, Klassen J, Brown CB, Russell JA. Double high-dose therapy for Hodgkin's disease with dose-intensive cyclophosphamide, etoposide, and cisplatin (DICEP) prior to high-dose melphalan and autologous stem cell transplantation. Bone Marrow Transplant. 2000 Aug;26(4):383-8. doi: 10.1038/sj.bmt.1702541. PMID: 10982284.
- Vijay A, Duan Q, Henning JW, Duggan P, Daly A, Shafey M, Bahlis NJ, Stewart DA. High dose salvage therapy with dose intensive cyclophosphamide, etoposide and cisplatin may increase transplant rates for relapsed/refractory aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2013 Dec;54(12):2620-6. doi: 10.3109/10428194.2013.783211. Epub 2013 Apr 16. PMID: 23472969.
- Shafey M, Duan Q, Russell J, Duggan P, Balogh A, Stewart DA. Double high-dose therapy with dose-intensive cyclophosphamide, etoposide, cisplatin (DICEP) followed by high-dose melphalan and autologous stem cell transplantation for relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma. 2012 Apr;53(4):596-602. doi: 10.3109/10428194.2011.624227. PMID: 21929284.
- Gokmen A, Sahin U, Soydan E, Gokgoz Z, Okcu MK, Ozan U, Arslan O, Ilhan O, Ozcan M. Gemcitabine, Cisplatin, and Dexamethasone as a Salvage and Mobilization Chemotherapy Before Autologous Stem Cell Transplantation is Effective and Safe Outpatient Regimen in Relapsed and Refractory Hodgkin Lymphoma Patients. Clin Lymphoma Myeloma Leuk. 2022 Oct;22(10):e885-e892. doi: 10.1016/j.clml.2022.06.015. Epub 2022 Jul 1. PMID: 35927182..
- Sarmiento M, Ramírez P, Parody R, Salas MQ, Beffermann N, Jara V, Bertín P, Pizarro I, Lorca C, Rivera E, Galleguillos M, Ocqueteau M, Sánchez-Ortega I, Patiño B, Sureda A. Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy. Bone Marrow Transplant. 2018 Aug;53(8):960-966. doi: 10.1038/s41409-018-0117-5. Epub 2018 Feb 13. PMID: 29440738.
- Rajkumar SV. Clinical features, laboratory manifestations, and diagnosis of multiple myeloma. Up to Date 2018. Edited by Kyle RA, Connor RF.
- Ricciuti G, Falcone A, Cascavilla N, Martinelli G, Cerchione C. Autologous stem cell transplantation in multiple myeloma. Panminerva Med. 2020 Dec;62(4):220-224. doi: 10.23736/S0031-0808.20.04114-2. Epub 2020 Sep 21. PMID: 32955179.
- Piriyakhuntorn P, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, Norasetthada L. Outcomes of Non-Cryopreserved Versus Cryopreserved Peripheral Blood Stem Cells for Autologous Stem Cell Transplantation in Multiple Myeloma. Ann Transplant. 2020 Dec 11;25:e927084. doi: 10.12659/AOT.927084. PMID: 33303730; PMCID: PMC7737409.
- Wannesson L, Panzarella T, Mikhael J, Keating A. Feasibility and safety of autotransplants with noncryopreserved marrow or peripheral blood stem cells: a systematic review. Ann Oncol. 2007 Apr;18(4):623-32. doi: 10.1093/annonc/mdm069. Epub 2007 Mar 12. PMID: 17355952.
- Ramzi M, Zakerinia M, Nourani H, Dehghani M, Vojdani R, Haghighinejad H. Non-cryopreserved hematopoietic stem cell transplantation in multiple myeloma, a single center experience. Clin Transplant. 2012 Jan-Feb;26(1):117-22. doi: 10.1111/j.1399-0012.2011.01432.x. Epub 2011 Sep 15. PMID: 21919958.
- Kayal S, Sharma A, Iqbal S, Tejomurtula T, Cyriac SL, Raina V. High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: a single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells. Clin Lymphoma Myeloma Leuk. 2014 Apr;14(2):140-7. doi: 10.1016/j.clml.2013.09.001. Epub 2013 Sep 28. PMID: 24342104.
- Bekadja MA, Brahimi M, Osmani S, Arabi A, Bouhass R, Yafour N, Entasoltan B, Rasheed W, Attaf F. A simplified method for autologous stem cell transplantation in multiple myeloma. Hematol Oncol Stem Cell Ther. 2012;5(1):49-53. doi: 10.5144/1658-3876.2012.49. PMID: 22446610.
- Jasuja SK, Kukar (jasuja) N, Jain R, Bhateja A, Jasuja A, Jain R. A simplified method at lowest cost for autologous, non-cryopreserved, unmanipulated, peripheral hematopoietic stem cell transplant in multiple myeloma and non-Hodgkin's lymphoma: Asian scenario. J Clin Oncol. 2010; 28(15): ė18545
- Ruiz-Argüelles GJ, Gómez-Rangel D, Ruiz-Delgado GJ, Ruiz-Argüelles A, Pérez-Romano B, Rivadeneyra L. Results of an autologous noncryopreserved, unmanipulated peripheral blood hematopoietic stem cell transplant program: a single-institution, 10-year experience. Acta Haematol. 2003;110(4):179-83. doi: 10.1159/000074221. PMID: 14663161.
- Drozd-Sokołowska J, Gras L, Zinger N, Snowden JA, Arat M, Basak G, Pouli A, Crawley C, Wilson KMO, Tilly H, Byrne J, Bulabois CE, Passweg J, Ozkurt ZN, Schroyens W, Lioure B, Colorado Araujo M, Poiré X, Van Gorkom G, Gurman G, de Wreede LC, Hayden PJ, Beksac M, Schönland SO, Yakoub-Agha I. Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation-study on behalf of CMWP of the EBMT. Bone Marrow Transplant. 2022 Apr;57(4):633-640. doi: 10.1038/s41409-022-01592-y. Epub 2022 Feb 15. PMID: 35169284; PMCID: PMC8993690.
- Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020 Sep 28;10(9):94. doi: 10.1038/s41408-020-00359-2. PMID: 32989217; PMCID: PMC7523011.
- Akhtar S. High dose chemotherapy and autologous stem cell transplantation in relapsed or refractory Hodgkin lymphoma: Emerging questions, newer agents, and changing paradigm. Hematol Oncol Stem Cell Ther. 2017; 10(4): 272-276. doi: 10.1016/j.hemonc.2017.05.010. Epub 2017 Jun 13.
- Reid RM, Baran A, Friedberg JW, Phillips GL 2nd, Liesveld JL, Becker MW, Wedow L, Barr PM, Milner LA. Outpatient administration of BEAM conditioning prior to autologous stem cell transplantation for lymphoma is safe, feasible, and cost-effective. Cancer Med. 2016 Nov;5(11):3059-3067. doi: 10.1002/cam4.879. Epub 2016 Oct 3. PMID: 27699999; PMCID: PMC5119960.
- Akhtar S, Rauf SM, Elhassan TA, Maghfoor I. Outcome analysis of high-dose chemotherapy and autologous stem cell transplantation in adolescent and young adults with relapsed or refractory Hodgkin lymphoma. Ann Hematol. 2016 Sep;95(9):1521-35. doi: 10.1007/s00277-016-2736-5. Epub 2016 Jul 4. PMID: 27376363.
- Sirohi B, Cunningham D, Powles R, Murphy F, Arkenau T, Norman A, Oates J, Wotherspoon A, Horwich A. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008 Jul;19(7):1312-1319. doi: 10.1093/annonc/mdn052. Epub 2008 Mar 19. PMID: 18356139.
- Nieto Y, Gruschkus S, Valdez BC, Jones RB, Anderlini P, Hosing C, Popat U, Qazilbash M, Kebriaei P, Alousi A, Saini N, Srour S, Rezvani K, Ramdial J, Barnett M, Gulbis A, Shigle TL, Ahmed S, Iyer S, Lee H, Nair R, Parmar S, Steiner R, Dabaja B, Pinnix C, Gunther J, Cuglievan B, Mahadeo K, Khazal S, Chuang H, Champlin R, Shpall EJ, Andersson BS. Improved outcomes of high-risk relapsed Hodgkin lymphoma patients after high-dose chemotherapy: a 15-year analysis. Haematologica. 2022 Apr 1;107(4):899-908. doi: 10.3324/haematol.2021.278311. PMID: 33951890; PMCID: PMC8968895.
- Casadei B, Argnani L, Morigi A, Lolli G, Broccoli A, Pellegrini C, Nanni L, Stefoni V, Coppola PE, Carella M, Cavo M, Zinzani PL. Potential survival benefit for patients receiving autologous hematopoietic stem cell transplantation after checkpoint inhibitors for relapsed/refractory Hodgkin lymphoma: A real-life experience. Hematol Oncol. 2020 Dec;38(5):737-741. doi: 10.1002/hon.2803. Epub 2020 Sep 30. PMID: 32905626.
- Cazeau N, Cavalier K, Bhatt V, McElrath C, Lestrange N, Lachaud-Richard M, et al. Outpatient BEAM using daily etoposide and cytarabine with autologous hematopoietic stem cell transplantation for lymphoma is feasible and decreases inpatient length of stay. Blood. 2019; 134 (Supplement_1): 5830. doi: 10.1182/ blood-2019-127402
- Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM, Scott JG, Sutcliffe SB, Brandwein JM, Keating A. Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol. 1995 Feb;13(2):396-402. doi: 10.1200/JCO.1995.13.2.396. PMID: 7844600.
- Fernández-Jiménez MC, Canales MA, Ojeda E, de Bustos JG, Aguado MJ, Hernández-Navarro F. Salvage chemotherapy with mini-BEAM for relapsed or refractory Hodgkin's disease prior to autologous peripheral blood stem cell transplantation. Haematologica. 1999 Nov;84(11):1007-11. PMID: 10553161.
- Friend BD, Muhsen IN, Patel S, Hill LC, Lulla P, Ramos CA, Pingali SR, Kamble RT, John TD, Salem B, Bhar S, Doherty EE, Craddock J, Sasa G, Wu M, Wang T, Martinez C, Krance RA, Heslop HE, Carrum G. Rituximab as adjunctive therapy to BEAM conditioning for autologous stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant. 2022 Apr;57(4):579-585. doi: 10.1038/s41409-022-01599-5. Epub 2022 Feb 1. PMID: 35105965.
- Herrera AF, Palmer J, Adhikarla V, Yamauchi D, Poku EK, Bading J, Yazaki P, Dandapani S, Mei M, Chen R, Cao T, Karras N, McTague P, Nademanee A, Popplewell L, Sahebi F, Shively JE, Simpson J, Smith DL, Song J, Spielberger R, Tsai NC, Thomas SH, Forman SJ, Colcher D, Wu AM, Wong J, Smith E. Anti-CD25 radioimmunotherapy with BEAM autologous hematopoietic cell transplantation conditioning in Hodgkin lymphoma. Blood Adv. 2021 Dec 14;5(23):5300-5311. doi: 10.1182/bloodadvances.2021004981. PMID: 34638132; PMCID: PMC9153018.
- Duléry R, Lebras L, Choquet S, Di Blasi R, AL Jijakli AK, Heuberger L, et al; TEAM conditioning (thiotepa, etoposide, cytarabine, melphalan) prior to autologous hematopoietic stem cell transplantation for Hodgkin and non- Hodgkin lymphoma: Final results from a prospective multicenter study. Blood. 2019; 134 (Supplement_1): 786. doi: 10.1182/blood-2019-130651
- Ahmed T, Ciavarella D, Feldman E, Ascensao J, Hussain F, Engelking C, Gingrich S, Mittelman A, Coleman M, Arlin ZA. High-dose, potentially myeloablative chemotherapy and autologous bone marrow transplantation for patients with advanced Hodgkin's disease. Leukemia. 1989 Jan;3(1):19-22. PMID: 2642573.
- Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, Scott JG, McCrae J, Murray C, Pantalony D, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin's disease: importance of disease status at transplant. J Clin Oncol. 1993 Apr;11(4):704-11. doi: 10.1200/JCO.1993.11.4.704. PMID: 8478664.
- Seymour LK, Dansey RD, Bezwoda WR. Single high-dose etoposide and melphalan with non-cryopreserved autologous marrow rescue as primary therapy for relapsed, refractory and poor-prognosis Hodgkin's disease. Br J Cancer. 1994 Sep;70(3):526-30. doi: 10.1038/bjc.1994.339. PMID: 8080741; PMCID: PMC2033336.
- Taylor PR, Jackson GH, Lennard AL, Lucraft H, Proctor SJ. Autologous transplantation in poor risk Hodgkin's disease using high dose melphalan/etoposide conditioning with non-cryopreserved marrow rescue. The Newcastle and Northern Region Lymphoma Group. Br J Cancer. 1993 Feb;67(2):383-7. doi: 10.1038/bjc.1993.70. PMID: 8431371; PMCID: PMC1968195.
- Isidori A, Christofides A, Visani G. Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: alternatives to BEAM conditioning. Leuk Lymphoma. 2016 Nov;57(11):2499-509. doi: 10.1080/10428194.2016.1185785. Epub 2016 May 31. PMID: 27243412.
- Okay M, Büyükaşık Y, Demiroğlu H, Malkan ÜY, Çiftçiler R, Aladağ E, Aksu S, Haznedaroğlu İC, Sayınalp N, Özcebe Oİ, Göker H. Mitoxantrone-melphalan conditioning regimen for autologous stem cell transplantation in relapsed/refractory lymphoma. Turk J Med Sci. 2019 Aug 8;49(4):985-992. doi: 10.3906/sag-1809-36. PMID: 31293116; PMCID: PMC7018231.
- Yeral M, Aytan P, Gungor B, Boga C, Unal A, Koc Y, Kaynar L, Buyukkurt N, Eser B, Ozdoğu H. A Comparison of the BEAM and MITO/MEL Conditioning Regimens for Autologous Hematopoietic Stem Cell Transplantation in Hodgkin Lymphoma: An Analysis of Efficiency and Treatment-Related Toxicity. Clin Lymphoma Myeloma Leuk. 2020 Oct;20(10):652-660. doi: 10.1016/j.clml.2020.05.009. Epub 2020 May 21. PMID: 32605899.
- Fernández-Gutiérrez JA, Reyes-Cisneros OA, Litzow MR, Bojalil-Alvarez L, Garcia-Villasenor E, Gómez-Gomez ET, Murrieta-Alvarez I, Gomez-Almaguer D, Gutierrez-Aguirre CH, Karduss-Urueta AJ, Ruiz-Delgado GJ, Ruiz-Arguelles GJ. High dose melphalan is an adequate preparative regimen for autologous hematopoietic stem cell transplantation in relapsed/refractory lymphoma. Hematology. 2022 Dec;27(1):449-455. doi: 10.1080/16078454.2022.2059630. PMID: 35413225.
- Kaloyannidis P, Al Hashmi H , Rauf MS, Maghfoor I , Harbi S, Kafnar S, et al. BEAM versus single agent high dose melphalan (HDM) conditioning regimen for autologous hematopoietic stem cell transplant (ASCT): a retrospective matched analysis in relapse/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019; 25 (3): S187-S188. Doi: 10.1016/j.bbmt.2018.12.773
- Al Hashmi H, Kaloyannidis P, Kafnar S, Al Harbi S, Shaibani E, Mokhtar N, et al. Single-agent high-dose melphalan as conditioning regimen in autologous hematopoietic stem cell transplantation for Hodgkin's lymphoma: safety, and long-term efficacy. Biol Blood Marrow Transplant. 2018; 24(3): S132–133. doi: 10.1016/j.bbmt.2017. 12.076. Epub 2018 Feb 3.
- Russell JA, Selby PJ, Ruether BA, Mbidde EK, Ashley S, Zulian G, Berry J, Houwen B, Jones AR, Poon MC, et al. Treatment of advanced Hodgkin's disease with high dose melphalan and autologous bone marrow transplantation. Bone Marrow Transplant. 1989 Jul;4(4):425-9. PMID: 2673463.
- Kopińska A, Koclęga A, Wieczorkiewicz-Kabut A, Woźniczka K, Kata D, Włodarczyk M, Helbig G. Allogeneic Stem Cell Transplantation for Relapsed and Refractory Hodgkin Lymphoma: Real World Experience of a Single Center. Pathol Oncol Res. 2021 Jul 27;27:1609867. doi: 10.3389/pore.2021.1609867. PMID: 34385892; PMCID: PMC8354297.
- Graff TM, Singavi AK, Schmidt W, Eastwood D, Drobyski WR, Horowitz M, Palmer J, Pasquini M, Rizzo DJ, Saber W, Hari P, Fenske TS. Safety of outpatient autologous hematopoietic cell transplantation for multiple myeloma and lymphoma. Bone Marrow Transplant. 2015 Jul;50(7):947-53. doi: 10.1038/bmt.2015.46. Epub 2015 Apr 13. PMID: 25867651; PMCID: PMC4490016.
- Larsen K, Spencer H, Mohan M, Bailey C, Hill K, Kottarathara M, Parikh R, Hoque S, Erra A, Mitma AA, Mathur P, Yarlagadda L, Gundarlapalli S, Ogunsesan Y, Hussain M, Thalambedu N, Sehti J, Al Hadidi S, Thanendrarajan S, Graziutti M, Zangari M, Barlogie B, van Rhee F, Tricot G, Schinke C. Feasibility of Outpatient Stem Cell Transplantation in Multiple Myeloma and Risk Factors Predictive of Hospital Admission. J Clin Med. 2022 Mar 16;11(6):1640. doi: 10.3390/jcm11061640. PMID: 35329966; PMCID: PMC8955129.
- Leger C, Sabloff M, McDiarmid S, Bence-Bruckler I, Atkins H, Bredeson C, Zhang H, Huebsch L. Outpatient autologous hematopoietic stem cell transplantation for patients with relapsed follicular lymphoma. Ann Hematol. 2006 Oct;85(10):723-9. doi: 10.1007/s00277-006-0149-6. Epub 2006 Jul 11. PMID: 16832675.
- Peters WP, Ross M, Vredenburgh JJ, Hussein A, Rubin P, Dukelow K, Cavanaugh C, Beauvais R, Kasprzak S. The use of intensive clinic support to permit outpatient autologous bone marrow transplantation for breast cancer. Semin Oncol. 1994 Aug;21(4 Suppl 7):25-31. PMID: 7916487.
- Koo J, Silverman S, Nuechterlein B, Keating AK, Verneris MR, Foreman NK, Mulcahy Levy JM. Safety and feasibility of outpatient autologous stem cell transplantation in pediatric patients with primary central nervous system tumors. Bone Marrow Transplant. 2019 Oct;54(10):1605-1613. doi: 10.1038/s41409-019-0479-3. Epub 2019 Feb 19. PMID: 30783209; PMCID: PMC6957458.
- Campbell P, Walker PA, Avery S, Patil SS, Curtis DJ, Schwarer AP, et al. Outpatient non-myeloablative allogeneic stem cell transplantation for myeloma is feasible, efficacious and associated with low transplant-related morbidity and mortality. Blood 2013; 122 (21): 2128. doi: 10.1182/blood. V122.21.2128.2128
- Campbell P, Walker P, Avery S, Patil S, Curtis D, Schwarer A, Wei A, Kalff A, Muirhead J, Spencer A. Safe and effective use of outpatient non-myeloablative allogeneic stem cell transplantation for myeloma. Blood Cancer J. 2014 May 9;4(5):e213. doi: 10.1038/bcj.2014.33. PMID: 24813081; PMCID: PMC4042303.
- Gutiérrez-Aguirre CH, Ruiz-Argüelles G, Cantú-Rodríguez OG, González-Llano O, Jaime-Pérez JC, García-Rodríguez F, López-Otero A, Herrera-Garza JL, Gómez-Almaguer D. Outpatient reduced-intensity allogeneic stem cell transplantation for patients with refractory or relapsed lymphomas compared with autologous stem cell transplantation using a simplified method. Ann Hematol. 2010 Oct;89(10):1045-52. doi: 10.1007/s00277-010-0986-1. Epub 2010 May 21. PMID: 20490794.
- Purev E, Srinivasan R, Aue G, Stroncek D, Khuu H, Ramos C, et al. Outpatient allogeneic hematopoietic cell transplant following alemtuzumab based reduced intensity conditioning in patients with advanced mycosis fungoides/Sezary syndrome. J Clin Oncol. 2015; 33: 15_suppl, 7089-7089. doi: 10.1200/jco.2015.33.15_suppl. 7089
- Gómez-Almaguer D, Gómez-De León A, Colunga-Pedraza PR, Cantú-Rodríguez OG, Gutierrez-Aguirre CH, Ruíz-Arguelles G. Outpatient allogeneic hematopoietic stem-cell transplantation: a review. Ther Adv Hematol. 2022 Feb 26;13:20406207221080739. doi: 10.1177/20406207221080739. PMID: 35237396; PMCID: PMC8882949.
- Cantú-Rodríguez OG, Sánchez-Cárdenas M, Treviño-Montemayor OR, Gutiérrez-Aguirre CH, Tarín-Arzaga L, Jaime-Pérez JC, Gómez-Almaguer D. Impact of outpatient non-myeloablative haematopoietic stem cell transplantation in quality of life vs. conventional therapy. Psychol Health Med. 2016;21(1):10-9. doi: 10.1080/13548506.2015.1054843. Epub 2015 Jun 30. PMID: 26125120.
- Rives S, Carreras E, Rovira M, Montoto S, Urbano-Ispizua A, Martínez C, Perales M, Alvarez A, Esteve J, González M, Montserrat E. Trasplante autogénico de progenitores hemopoyéticos en régimen ambulatorio: análisis de viabilidad en el Hospital Clínic de Barcelona [The autologous transplantation of hematopoietic precursors on an outpatient basis: an analysis of its feasibility at the Hospital Clínic de Barcelona]. Med Clin (Barc). 1999 Sep 4;113(6):201-4. Spanish. PMID: 10472607.
- Dytfeld D, Łojko-Dankowska A, Nowicki A, Matuszak M, Wache A, Gil L. Safety and cost effectiveness of outpatient autologous hematopoietic stem cell transplantation for multiple myeloma - single-center experience of a pilot Early Discharge Program. Acta Haematol Pol 2021; 52(3): 178-181. doi: 10.5603/ AHP. a021.0029·
- Martino M, Lemoli RM, Girmenia C, Castagna L, Bruno B, Cavallo F, Offidani M, Scortechini I, Montanari M, Milone G, Postacchini L, Olivieri A. Italian consensus conference for the outpatient autologous stem cell transplantation management in multiple myeloma. Bone Marrow Transplant. 2016 Aug;51(8):1032-40. doi: 10.1038/bmt.2016.79. Epub 2016 Apr 4. PMID: 27042841.
- Martino M, Montanari M, Bruno B, Console G, Irrera G, Messina G, Offidani M, Scortechini I, Moscato T, Fedele R, Milone G, Castagna L, Olivieri A. Autologous hematopoietic progenitor cell transplantation for multiple myeloma through an outpatient program. Expert Opin Biol Ther. 2012 Nov;12(11):1449-62. doi: 10.1517/14712598.2012.707185. Epub 2012 Jul 13. PMID: 22788745.
- Tan XN, Yew CY, Ragg SJ, Harrup RA, Johnston AM. Outpatient autologous stem cell transplantation in Royal Hobart Hospital, Tasmania: a single-centre, retrospective review in the Australian setting. Intern Med J. 2022 Jul;52(7):1242-1250. doi: 10.1111/imj.15334. Epub 2022 May 31. PMID: 33949777.
- Jaime-Pérez JC, Hernández-Coronado M, Picón-Galindo E, Salazar-Cavazos L, Gutiérrez-Aguirre CH, Gómez-Almaguer D. Results of a completely outpatient autologous stem cell transplant program for lymphoma patients receiving reduced-intensity conditioning. Leuk Lymphoma. 2021 Jul;62(7):1619-1628. doi: 10.1080/10428194.2021.1876870. Epub 2021 Jan 24. PMID: 33491518.
- Owattanapanich W, Suphadirekkul K, Kunacheewa C, Ungprasert P, Prayongratana K. Risk of febrile neutropenia among patients with multiple myeloma or lymphoma who undergo inpatient versus outpatient autologous stem cell transplantation: a systematic review and meta-analysis. BMC Cancer. 2018 Nov 16;18(1):1126. doi: 10.1186/s12885-018-5054-6. PMID: 30445930; PMCID: PMC6240267.
- Martino M, Paviglianiti A, Memoli M, Martinelli G, Cerchione C. Multiple Myeloma Outpatient Transplant Program in the Era of Novel Agents: State-of-the-Art. Front Oncol. 2020 Nov 11;10:592487. doi: 10.3389/fonc.2020.592487. PMID: 33262948; PMCID: PMC7686536.
- Abid MB, Christopher D, Abid MA, Poon ML, Tan LK, Koh LP, Chng WJ. Safety and cost-effectiveness of outpatient autologous transplantation for multiple myeloma in Asia: single-center perspective from Singapore. Bone Marrow Transplant. 2017 Jul;52(7):1044-1046. doi: 10.1038/bmt.2017.77. Epub 2017 May 8. PMID: 28481354.
- Obiozor C, Subramaniam DP, Divine C, Shune L, Singh AK, Lin TL, Abhyankar S, Chen GJ, McGuirk J, Ganguly S. Evaluation of Performance Status and Hematopoietic Cell Transplantation Specific Comorbidity Index on Unplanned Admission Rates in Patients with Multiple Myeloma Undergoing Outpatient Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017 Oct;23(10):1641-1645. doi: 10.1016/j.bbmt.2017.06.001. Epub 2017 Jun 8. PMID: 28603071.
- Martino M, Console G, Russo L, Meliado' A, Meliambro N, Moscato T, Irrera G, Messina G, Pontari A, Morabito F. Autologous Stem Cell Transplantation in Patients With Multiple Myeloma: An Activity-based Costing Analysis, Comparing a Total Inpatient Model Versus an Early Discharge Model. Clin Lymphoma Myeloma Leuk. 2017 Aug;17(8):506-512. doi: 10.1016/j.clml.2017.05.018. Epub 2017 Jun 6. PMID: 28647402.
- Kodad SG, Sutherland H, Limvorapitak W, Abou Mourad Y, Barnett MJ, Forrest D, Gerrie A, Hogge DE, Nantel SH, Narayanan S, Nevill T, Power M, Sanford D, Toze C, White J, Broady R, Song K. Outpatient Autologous Stem Cell Transplants for Multiple Myeloma: Analysis of Safety and Outcomes in a Tertiary Care Center. Clin Lymphoma Myeloma Leuk. 2019 Dec;19(12):784-790. doi: 10.1016/j.clml.2019.09.619. Epub 2019 Oct 9. PMID: 31678079.
- Gertz MA, Ansell SM, Dingli D, Dispenzieri A, Buadi FK, Elliott MA, Gastineau DA, Hayman SR, Hogan WJ, Inwards DJ, Johnston PB, Kumar S, Lacy MQ, Leung N, Micallef IN, Porrata LF, Schafer BA, Wolf RC, Litzow MR. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008 Oct;83(10):1131-8. doi: 10.4065/83.10.1131. PMID: 18828972.
- Khouri J, Majhail NS. Advances in delivery of ambulatory autologous stem cell transplantation for multiple myeloma. Curr Opin Support Palliat Care. 2017 Dec;11(4):361-365. doi: 10.1097/SPC.0000000000000305. PMID: 28922292.
- Marini J, Maldonado A, Weeda ER, Hashmi H, Neppalli AK, Edwards K. Efficacy, safety and cost implications of outpatient autologous hematopoietic stem cell transplant for multiple myeloma: a single center experience. Blood. 2020; 136 (Suppl.1): 31. doi: 10.1182/blood-2020-143137
- Lisenko K, Sauer S, Bruckner T, Egerer G, Goldschmidt H, Hillengass J, Schmier JW, Shah S, Witzens-Harig M, Ho AD, Wuchter P. High-dose chemotherapy and autologous stem cell transplantation of patients with multiple myeloma in an outpatient setting. BMC Cancer. 2017 Feb 22;17(1):151. doi: 10.1186/s12885-017-3137-4. PMID: 28228122; PMCID: PMC5322605.
- Jagannath S, Vesole DH, Zhang M, Desikan KR, Copeland N, Jagannath M, Bracy D, Jones R, Crowley J, Tricot G, Barlogie B. Feasibility and cost-effectiveness of outpatient autotransplants in multiple myeloma. Bone Marrow Transplant. 1997 Sep;20(6):445-50. doi: 10.1038/sj.bmt.1700900. PMID: 9313876.
- Martino M, Russo L, Martinello T, Gallo GA, Fedele R, Moscato T, Console G, Vincelli DI, Ronco F, Postorino M, Irrera G, Messina G. A home-care, early discharge model after autografting in multiple myeloma: results of a three-arm prospective, non-randomized study. Leuk Lymphoma. 2015 Mar;56(3):801-4. doi: 10.3109/10428194.2014.931952. Epub 2014 Jul 17. PMID: 24913501.
- Ferrara F, Izzo T, Criscuolo C, Riccardi C, Viola A, Delia R, Carbone A, Celentano M. Comparison of fixed dose pegfilgrastim and daily filgrastim after autologous stem cell transplantation in patients with multiple myeloma autografted on a outpatient basis. Hematol Oncol. 2011 Sep;29(3):139-43. doi: 10.1002/hon.978. Epub 2010 Nov 30. PMID: 21922508.
- Holbro A, Ahmad I, Cohen S, Roy J, Lachance S, Chagnon M, LeBlanc R, Bernard L, Busque L, Roy DC, Sauvageau G, Kiss TL. Safety and cost-effectiveness of outpatient autologous stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2013 Apr;19(4):547-51. doi: 10.1016/j.bbmt.2012.12.006. Epub 2012 Dec 16. PMID: 23253556.
- Paul TM, Liu SV, Chong EA, Luger SM, Porter DL, Schuster SJ, Tsai DE, Nasta SD, Loren A, Frey N, Perl A, Cohen AD, Weiss BM, Stadtmauer EA, Vogl DT. Outpatient Autologous Stem Cell Transplantation for Patients With Myeloma. Clin Lymphoma Myeloma Leuk. 2015 Sep;15(9):536-40. doi: 10.1016/j.clml.2015.05.006. Epub 2015 Jun 6. PMID: 26141214.
- Son T, Lambert S, Jakubowski A, DiCicco-Bloom B, Loiselle CG. Adaptation of Coping Together - a self-directed coping skills intervention for patients and caregivers in an outpatient hematopoietic stem cell transplantation setting: a study protocol. BMC Health Serv Res. 2018 Aug 29;18(1):669. doi: 10.1186/s12913-018-3483-1. PMID: 30157867; PMCID: PMC6114732.
- Kodad SG, Sutherland H, Limvorapitak W, Abou Mourad Y, Barnett MJ, Forrest D, Gerrie A, Hogge DE, Nantel SH, Narayanan S, Nevill T, Power M, Sanford D, Toze C, White J, Broady R, Song K. Outpatient Autologous Stem Cell Transplants for Multiple Myeloma: Analysis of Safety and Outcomes in a Tertiary Care Center. Clin Lymphoma Myeloma Leuk. 2019 Dec;19(12):784-790. doi: 10.1016/j.clml.2019.09.619. Epub 2019 Oct 9. PMID: 31678079.
- Frey P, Stinson T, Siston A, Knight SJ, Ferdman E, Traynor A, O'Gara K, Rademaker A, Bennett C, Winter JN. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant. 2002 Dec;30(11):741-8. doi: 10.1038/sj.bmt.1703676. PMID: 12439696.
- Faucher C, Le Corroller Soriano AG, Esterni B, Vey N, Stoppa AM, Chabannon C, Mohty M, Michallet M, Bay JO, Genre D, Maraninchi D, Viens P, Moatti JP, Blaise D. Randomized study of early hospital discharge following autologous blood SCT: medical outcomes and hospital costs. Bone Marrow Transplant. 2012 Apr;47(4):549-55. doi: 10.1038/bmt.2011.126. Epub 2011 Jul 4. PMID: 21725375.
- Ferrara F, Palmieri S, Viola A, Copia C, Schiavone EM, De Simone M, Pocali B, D'Amico MR, Annunziata M, Mele G. Outpatient-based peripheral blood stem cell transplantation for patients with multiple myeloma. Hematol J. 2004;5(3):222-6. doi: 10.1038/sj.thj.6200349. PMID: 15167908.
- Meisenberg BR, Ferran K, Hollenbach K, Brehm T, Jollon J, Piro LD. Reduced charges and costs associated with outpatient autologous stem cell transplantation. Bone Marrow Transplant. 1998 May;21(9):927-32. doi: 10.1038/sj.bmt.1701191. PMID: 9613786.
- Fernández-Avilés F, Carreras E, Urbano-Ispizua A, Rovira M, Martínez C, Gaya A, Granell M, Ramiro L, Gallego C, Hernando A, Segura S, García L, González M, Valverde M, Montserrat E. Case-control comparison of at-home to total hospital care for autologous stem-cell transplantation for hematologic malignancies. J Clin Oncol. 2006 Oct 20;24(30):4855-61. doi: 10.1200/JCO.2006.06.4238. Epub 2006 Sep 25. PMID: 17001069.
- Vaxman I, Gertz M. Risk adapted post-transplant maintenance in multiple myeloma. Expert Rev Hematol. 2019 Feb;12(2):107-118. doi: 10.1080/17474086.2019.1576521. PMID: 30696304.
- Goldschmidt H, Mai EK, Dürig J, Scheid C, Weisel KC, Kunz C, Bertsch U, Hielscher T, Merz M, Munder M, Lindemann HW, Hügle-Dörr B, Tichy D, Giesen N, Hose D, Seckinger A, Huhn S, Luntz S, Jauch A, Elmaagacli A, Rabold B, Fuhrmann S, Brossart P, Goerner M, Bernhard H, Hoffmann M, Hillengass J, Raab MS, Blau IW, Hänel M, Salwender HJ; German-speaking Myeloma Multicenter Group (GMMG). Response-adapted lenalidomide maintenance in newly diagnosed myeloma: results from the phase III GMMG-MM5 trial. Leukemia. 2020 Jul;34(7):1853-1865. doi: 10.1038/s41375-020-0724-1. Epub 2020 Feb 7. PMID: 32034285.
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L, Decaux O, Leyvraz S, Vekemans MC, Voillat L, Michallet M, Pegourie B, Dumontet C, Roussel M, Leleu X, Mathiot C, Payen C, Avet-Loiseau H, Harousseau JL; IFM Investigators. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1782-91. doi: 10.1056/NEJMoa1114138. PMID: 22571202.
- McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, Bringhen S, Musto P, Anderson KC, Caillot D, Gay F, Moreau P, Marit G, Jung SH, Yu Z, Winograd B, Knight RD, Palumbo A, Attal M. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017 Oct 10;35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679. Epub 2017 Jul 25. PMID: 28742454; PMCID: PMC5652871.
- Syed YY. Lenalidomide: A Review in Newly Diagnosed Multiple Myeloma as Maintenance Therapy After ASCT. Drugs. 2017 Sep;77(13):1473-1480. doi: 10.1007/s40265-017-0795-0. PMID: 28791622.
- Sivaraj D, Green MM, Li Z, Sung AD, Sarantopoulos S, Kang Y, Long GD, Horwitz ME, Lopez RD, Sullivan KM, Rizzieri DA, Chao NJ, Gasparetto C. Outcomes of Maintenance Therapy with Bortezomib after Autologous Stem Cell Transplantation for Patients with Multiple Myeloma. Biol Blood Marrow Transplant. 2017 Feb;23(2):262-268. doi: 10.1016/j.bbmt.2016.11.010. Epub 2016 Nov 14. PMID: 27856369.
- Sahebi F, Frankel PH, Farol L, Krishnan AY, Cai JL, Somlo G, Thomas SH, Reburiano E, Popplewell LL, Parker PM, Spielberger RT, Kogut NM, Karanes C, Htut M, Ruel C, Duarte L, Murata-Collins JL, Forman SJ. Sequential bortezomib, dexamethasone, and thalidomide maintenance therapy after single autologous peripheral stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2012 Mar;18(3):486-92. doi: 10.1016/j.bbmt.2011.12.580. Epub 2011 Dec 22. PMID: 22198542..
- Baertsch MA, Mai EK, Hielscher T, Bertsch U, Salwender HJ, Munder M, Fuhrmann S, Dührsen U, Brossart P, Neben K, Schlenzka J, Kunz C, Raab MS, Hillengaß J, Jauch A, Seckinger A, Hose D, Luntz S, Sonneveld P, Lokhorst H, Martin H, Goerner M, Hoffmann M, Lindemann HW, Bernhard H, Blau IW, Scheid C, Besemer B, Weisel KC, Hänel M, Dürig J, Goldschmidt H; German-Speaking Myeloma Multicenter Group (GMMG). Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2021 Jan 7;11(1):1. doi: 10.1038/s41408-020-00390-3. PMID: 33414374; PMCID: PMC7791127.
- Bonello F, Cetani G, Bertamini L, Gay F, Larocca A. Moving Toward Continuous Therapy in Multiple Myeloma. Clin Hematol Int. 2019 Nov 12;1(4):189-200. doi: 10.2991/chi.d.191101.001. PMID: 34595430; PMCID: PMC8432368.
- Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Gianni AM, Carella A, Osmanov D, Bachanova V, Sweetenham J, Sureda A, Huebner D, Sievers EL, Chi A, Larsen EK, Hunder NN, Walewski J; AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015 May 9;385(9980):1853-62. doi: 10.1016/S0140-6736(15)60165-9. Epub 2015 Mar 19. Erratum in: Lancet. 2015 Aug 8;386(9993):532. Erratum in: Lancet. 2015 Aug 8;386(9993):532. PMID: 25796459.
- Akay OM, Ozbalak M, Pehlivan M, Yildiz B, Uzay A, Yigenoglu TN, Elverdi T, Kaynar L, Ayyildiz O, Yonal Hindilerden I, Goksoy HS, Izmir Guner S, Gunes AK, Sonmez M, Kurt Yuksel M, Civriz Bozdag S, Ozkurt ZN, Toptas T, Dogu MH, Salim O, Saydam G, Yavasoglu I, Ayli M, Ozet G, Albayrak M, Birtas Atesoglu E, Toprak SK, Yildirim R, Mehtap O, Kalayoglu Besisik S, Nalcaci M, Altuntas F, Ferhanoglu B. Brentuximab vedotin consolidation therapy after autologous stem-cell transplantation in patients with high-risk Hodgkin lymphoma: Multicenter retrospective study. Hematol Oncol. 2021 Oct;39(4):498-505. doi: 10.1002/hon.2897. Epub 2021 Jun 25. PMID: 34171130.
- Marouf A, Cottereau AS, Kanoun S, Deschamps P, Meignan M, Franchi P, Sibon D, Antoine C, Gastinne T, Borel C, Hammoud M, Sicard G, Gille R, Cavalieri D, Stamatoullas A, Filliatre-Clement L, Lazarovici J, Chauchet A, Fornecker LM, Amorin S, Rocquet M, Raus N, Burroni B, Rubio MT, Bouscary D, Quittet P, Casasnovas RO, Brice P, Ghesquieres H, Tamburini J, Deau B. Outcomes of refractory or relapsed Hodgkin lymphoma patients with post-autologous stem cell transplantation brentuximab vedotin maintenance: a French multicenter observational cohort study. Haematologica. 2022 Jul 1;107(7):1681-1686. doi: 10.3324/haematol.2021.279564. PMID: 34965701; PMCID: PMC9244814.
- Sureda A, André M, Borchmann P, da Silva MG, Gisselbrecht C, Vassilakopoulos TP, Zinzani PL, Walewski J. Improving outcomes after autologous transplantation in relapsed/refractory Hodgkin lymphoma: a European expert perspective. BMC Cancer. 2020 Nov 10;20(1):1088. doi: 10.1186/s12885-020-07561-2. PMID: 33172440; PMCID: PMC7657361.
- Bonthapally V, Wu E, Macalalad A, Yang H, Shonukan O, Liu Y, Chi A, Huebner D. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous transplant: meta-analysis versus historical data. Curr Med Res Opin. 2015 May;31(5):993-1001. doi: 10.1185/03007995.2015.1030378. Epub 2015 Apr 9. PMID: 25772232.
- Riedell PA, Bishop MR. Post-autologous transplant maintenance therapies in lymphoma: current state and future directions. Bone Marrow Transplant. 2018 Jan;53(1):11-21. doi: 10.1038/bmt.2017.196. Epub 2017 Oct 2. PMID: 28967896.