More Information
Submitted: March 15, 2024 | Approved: April 30, 2024 | Published: May 01, 2024
How to cite this article: Casadei A, Gennai A, Bovani B, Sileo L, Cavaleri MP, et al. The Healing and Aging-related Properties of Adipose Tissue Fragments Obtained through the Guided SEFFI Procedure’s Mechanical Fragmentation are Facilitated by the Exosomes Present in the Final Injection. J Stem Cell Ther Transplant. 2024; 8: 010-015.
DOI: 10.29328/journal.jsctt.1001037
Copyright License: © 2024 Casadei A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Fat grafting; Soft-tissue augmentation; Soft-tissue reconstruction; Regenerative medicine; ADSCs; Exosomes
The Healing and Aging-related Properties of Adipose Tissue Fragments Obtained through the Guided SEFFI Procedure’s Mechanical Fragmentation are Facilitated by the Exosomes Present in the Final Injection
Alessandro Casadei1, Alessandro Gennai2, Bruno Bovani3, Lucia Sileo4, Maria Pia Cavaleri4, Martina Greco4 and Barbara Zavan4*
1Villa Salus Hospital and Casadei Clinic, Venezia-Mestre (VE), Italy
2Studio Gennai, Bologna (BO), Italy
3Esculapio Clinic, Perugia (PG), Republic of San Marino, Italy
4Department of Translational Medicine, University of Ferrara, via Fossato di Mortara 70, 44121 Ferrara, Italy
*Address for Correspondence: Barbara Zavan, Department of Translational Medicine, University of Ferrara, via Fossato di Mortara 70, 44121 Ferrara, Italy, Email: [email protected]
The Injection of autologous Adipose-Derived Stem Cells (ADSCs) and Stromal Vascular Fraction (SVF) into dermal and subdermal layers can improve skin volume and rejuvenation. The SEFFI (Superficial Enhanced Fluid Fat Injection) technique, which involves minimal manipulation of autologous microfragmented adipose tissue, was utilized for harvesting and re-injection, using the SEFFILLER™ disposable medical device. Mechanical fragmentation of adipose tissue is a well-established surgical technique that stimulates tissue regeneration, filler, and biological activity. The study evaluated the biological properties (regenerative and anti-aging) of different harvest and processing fat graft methods among which the fragmented adipose tissue, specifically focusing on the presence of exosomes. Exosomes, nanometer-sized vesicles produced by cells for cellular communication, were found to contain miRNAs with anti-inflammatory, regenerative, and vascular content. The products’ contained exosomes were confirmed in the study through electron microscopy, Western Blotting, gene expression, and sequencing of miRNA content.
The in vitro treatment of fibroblasts with exosomes obtained by this fragmentation technique showed a 23% greater increase in the production of hyaluronic acid, type I collagen, and elastin compared to other techniques. This research highlights the potential regenerative and anti-aging properties of exosomes obtained through mechanical fragmentation of adipose tissue for skin rejuvenation and skin repair.
The first use of autologous fat grafts was presented by Neuber in 1893 for the treatment of facial defects [1]. Subsequently, since the 1980s, lipoaspirate transplant procedures have been codified, and they have become common in cosmetic and reconstructive surgery clinical practice. Different technical variants and clinical applications have opened the horizons to unexpected perspectives, pushing surgical activity and research toward the frontiers of regenerative medicine [2]. Regenerative medicine is a new treatment that has been developed to repair damaged tissue. This emerging field requires a reliable source of stem cells to heal and support the regeneration of damaged tissue. Adipose tissue stem cells are currently being considered an ideal source of Autologous Mesenchymal Stem Cells (ADSCs) [3], for clinical purposes, especially because they are easily obtained by liposuction under local anesthesia causing minimal discomfort to the patient compared to traditional methods of obtaining them [4].
ADSCs have been shown to have the ability to self-renew and have a high potential to differentiate into various specialized cell lineages from other tissues both in vivo and in vitro [5,6]. They also have trophic, antifibrotic, and immunomodulatory properties [7] and they can secrete bioactive molecules that stimulate angiogenesis and revascularization of fat grafts [8,9]. Therefore, they can be considered an ideal cell population for several clinical applications [10]. Indeed, adipose tissue and ADSCs have been successfully used in various regenerative therapies, such as bone and cartilage defects [11], chronic wounds, skin repairs, and all soft tissue trauma lesions, tumor resections, and congenital malformations [12,13].
Recently, ADSCs isolated from liposuction aspirate have also been considered for tissue augmentation surgeries, including antiaging medicine [8,14-17]. Due to the unpredictability of graft survival and the risk of nodules, many authors have focused on microfragmented adipose tissue grafts (collectively known as Stromal Vascular Fraction -SVF, which contains about 30% - 40% ADSCs) [18] as a valid approach for volumization and skin regeneration.
Fat harvest, processing method, and grafting technique
Many studies have shown that the smaller the fat clusters injected and the more superficial tissue is taken, the better the results. How much does sampling, processing, and grafting affect clinical practice? Due to the reproducibility of the results, a highly standardized method must be used [19].
The Stromal Vascular Fraction (SVF) of adipose tissue contains many cells that form interconnected cell populations: adipocyte progenitors, pericytes, endothelial progenitor cells, and transit-amplifying cells [20]. As mentioned above, ADSCs can secrete bioactive molecules that stimulate angiogenesis and have antifibrotic, antiapoptotic, and immunomodulatory properties [21].
Moreover, SVF/ADSCs induce the secretion of cytokine and growth factors which promote angiogenesis and, thus, revascularization of fat grafts [22,23]. Such characteristics of SVF/ADSCs could account for some effects observed after adipose tissue implantation, such as improved skin trophism, accelerated closure of complex wounds or ulcers, and enhancement of skin appearance after damage from radiotherapy [24].
In 2015 one of the Authors [25] standardized the new tissue graft technique named Superficial Enhanced Fluid Fat Injection (SEFFI) to achieve skin enhancement and volume restoration of the face: these techniques use to harvest adipose tissue using microcannula with small sideport holes (0.3 - 0.5 and 0.8 mm) in a superficial adipose tissue layer (SAT) [26], aimed at grafting adipose tissue, including the SVFCs and ADSCs contained therein. Since 2015, several studies have been published showing that using a special cannula with small sideports holes, adipose tissue can be harvested and selected for small clusters of cells that do not require any manipulation to reduce the size of the cluster and fluidify the tissue [27].
The absence of substantial manipulation guarantees the maximum viability and stemness of the harvested tissue. In fact, grafts prepared using simple decantation contained the highest number of viable adipocytes [28].
Exosomes
Exosomes as known, are nanometer-sized vesicles (approximately 40 nm - 160 nm) produced by cells as a means of cellular communication [29]. They were first reported in 1989 by Johnstone, et al. [30].
They transport various proteins, nucleic acids, and lipids. Exosome reflects the cellular environment from which it originates and acts as a paracrine molecule that interacts with the Extracellular Matrix (ECM) and neighboring cells. Currently, exosomes have shown many potential clinical applications, including stem cell maintenance and plasticity, biomarkers, adjuvants in chemotherapy and drug delivery, and wound healing supplements that promote angiogenesis [31].
The molecular biology underlying exosome signaling is still developing and is not yet fully understood in the literature. Although one of the most studied exosomes is derived from adipose tissue, it is important to note that each tissue or cell from which it originates may have specific effects through a unique mechanism [32]. Exosome biogenesis begins with folding across the plasma membrane to form a Clathrin-Coated Vesicle (CCV) and then an early endosome. Subsequently, a second inward budding of the endosomal membrane occurs and produces a Multivesicular Body (MVB) containing exosomes. Finally, the MVB fuses with the plasma membrane, and exosomes are released into the extracellular space. In particular, They contain micro-RNA (miRNA), messenger-RNA (mRNA), transcription factors, membrane-trafficking proteins, antigen-presenting proteins, and bilayer lipid membrane peptides [33] with high anti-inflammatory, regenerative, and vascular content. MicroRNAs (miRNA) are small endogenous single-stranded non-coding RNA molecules and they act by recognizing specific mRNA targets that play a role in cell proliferation, apoptosis, and differentiation.
In cellular communication, the existing old concept involved direct contact between cells, an autocrine, paracrine, or endocrine signal mediated by free proteins, or free signals in the cytoplasm. However, since the mid-1990s exosomes have been found to play a vital role in intercellular communication and immunological function. These messengers are placed inside vesicles, so they are a sort of email with sender, recipient, and content based on these miRNAs.
Purpose
The study focused on the use of ADSCs and SVF for skin regeneration through injection into dermal and subdermal layers. The biological basis supporting the mechanical fragmentation of adipose tissue as a strategy for tissue regeneration, however, has not been fully defined. The cell viability assessed after mechanical fragmentation induces cellular stress and wall damage such that the in vivo success observed from a clinical point of view is not justified [25]. In this regard the study aimed to evaluate the regenerative and anti-aging properties of the final fragmented product, particularly focusing on the exosomes present.
Initially, it was believed that ADSCs promote tissue regeneration by differentiating into desired cell types, but poor engraftment rates were observed due to cell adhesion issues [34,35].
Another proposed mechanism is cellular paracrine effects mediated by exosomes, microvesicles, and apoptotic bodies (EVs), which have been shown to have regenerative effects in various conditions like cardiovascular disease, kidney injury, brain injury, and skin wound healing [36].
A randomized controlled clinical multicenter trial was conducted in 24 consecutive patients with facial aging (skin atrophy and volume loss), chronic wounds, and soft tissue post-traumatic lesions of the lower limbs with the goal of regenerating tissues. Patients meeting type 1 or type 2 diabetes mellitus, cardiovascular or neurologic disorders, chronic drug therapy, smoking, and previous abdominal surgery (laparotomy) were excluded. The study participants were recruited at the time of request and taken care of by three different surgeons. They were randomly divided into two groups, whose demographic characteristics were = Group A: (3 F – 9 M; average age 46.6 ys) Group B: (5 F – 7 M; average age 46.8 ys). Each patient, after informed consent obtained in accordance with the Declaration of Helsinki guidelines, underwent adipose tissue harvest into the selected donor site under local anesthesia [Cold Ringer’s lactate solution (100 mL) was mixed with lidocaine 2% (200 mg), Sodium Bicarbonate (5 mEq) and epinephrine (1 ml/1 mg) following a standardized protocol and injected with a ratio of 1:1 and all procedures were performed in AA’s medical facilities. Preferred harvesting sites were the suprapubic region, hip, trochanteric region, and inner knee. After aspiration, the fat was mixed with cold Ringer’s solution to rinse it from the anesthetic and to facilitate tissue precipitation.
Two different methods of collecting and processing adipose tissue were used. In Group A the adipose tissue was harvested with traditional liposuction technique using a cannula of 3mm diameter (Coleman) then it was centrifuged for 1 minute at 3500 rpm, In group B cannula of 2 mm diameter, 15 round side port holes of 0.8 mm diameter (SEFFI method) then it was left to decantation for 5 minutes. One sample of each patient, harvested from human adipose tissue during a surgical procedure, was sent to the laboratory.
The samples were subjected to evaluation of particle concentration per mL in the size spectrum between 100 and 200 nm and were characterized with C81 and CD63 markers and showed the shape of vesicles at Transmission Electron Microscopy (TEM). Finally, the direct effect on fibroblast gene expression crash tests was evaluated.
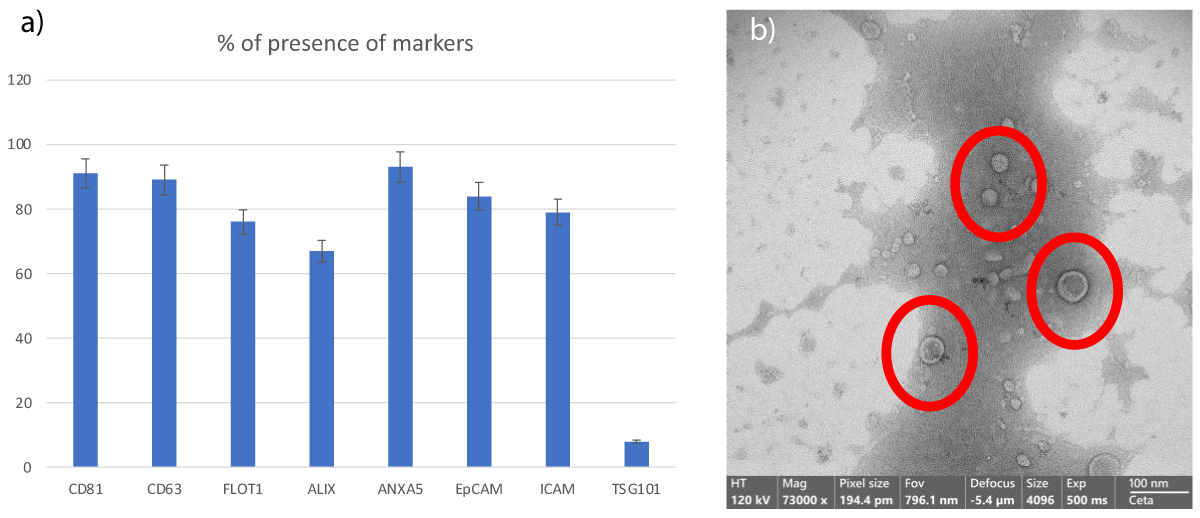
The comprehensive analysis conducted on the samples provides compelling evidence supporting their exosomal identity. Not only were specific molecular markers abundantly present on the surfaces of these exosomes, as indicated by sophisticated detection methods, but the meticulous examination via Transmission Electron Microscopy (TEM) further elucidated their nano-sized morphology, confirming their classification as exosomes with high fidelity (Figure 1). This multi-faceted characterization underscores the rigor and thoroughness of the investigative approach, instilling confidence in the veracity of the findings.
Figure 1: Figure 1: Analysis of the content of exosomes derived from microfragmented adipose tissue sampling with the SEFFI method:
(a) The histogram demonstrates the presence of specific exosomal proteins
(b) SEM images illustrate the size distribution of exos assessed also via Tunable Resistive Pulse Sensing (qNano) data do not show, revealing a mean diameter of 95 nm (Standard Deviation ± 35.6) and a typical shape, captured using the SEM Zeiss EVO 40 microscope (Zeiss, Oberkochen, Germany) at 70,000x magnification.
Moreover, the in-depth scrutiny of miRNA content within these exosomes uncovered a rich and diverse repertoire of regulatory molecules, whose roles in orchestrating cellular processes associated with aging and wound healing are well-documented (Figure 2). The abundance of these miRNAs within the exosomal cargo highlights their potential as potent mediators of therapeutic effects, capable of modulating intricate molecular pathways to promote tissue regeneration and mitigate age-related degenerative processes.
Figure 2: Categorization of miRNAs contained in exosomes by SEFFI autologous micro-fragmented adipose tissue.
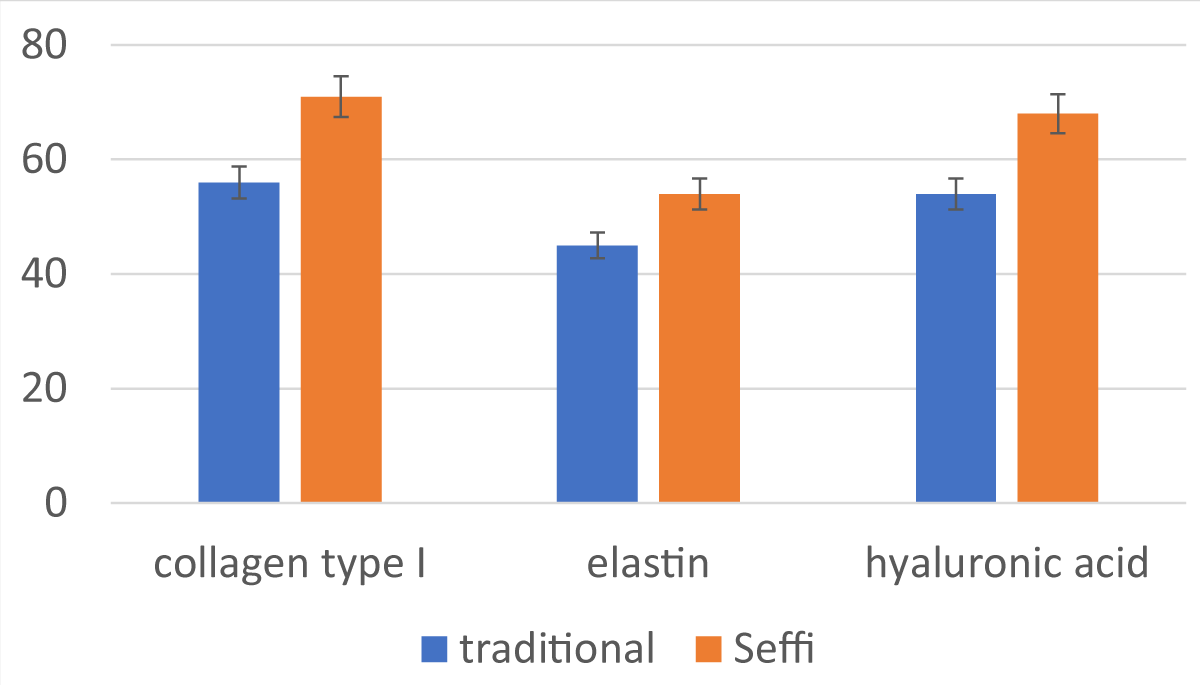
The translational implications of these findings are particularly noteworthy, especially concerning their application in clinical settings. The observed enhancement in gene expression related to essential extracellular matrix components—such as collagen, elastin, and hyaluronic acid—following in vitro treatment of fibroblasts with exosomes signifies a pivotal step forward in regenerative medicine and aesthetic dermatology (Figure 3). The augmentation of these crucial structural proteins holds immense promise for enhancing tissue elasticity, resilience, and overall quality, thereby presenting exciting avenues for addressing age-related changes and facilitating wound healing.
Figure 3: Trend of in vitro production of collagen, elastin, and hyaluronic acid in fibroblasts after application of exosomes obtained using different collection and processing systems and using the SEFFI method.
In summation, the comprehensive characterization of exosomes derived from adipose tissue fragments underscores their potential as potent therapeutic agents in combating the effects of aging and promoting tissue regeneration. The amalgamation of robust experimental methodologies, coupled with compelling translational outcomes, positions these exosomes as promising candidates for clinical applications aimed at rejuvenating tissues, ameliorating age-related ailments, and advancing the frontiers of regenerative medicine.
The purpose of the study was to evaluate the regenerative and anti-aging properties of the fragmented final product, focusing especially on the exosomes present. Therefore, clinical results related to the quality of tissue regeneration, where the primary endpoint was facial volume restoration, rhytids reduction, skin quality improvement, and success in more rapid and complete healing and repair of chronic wounds, and soft tissue trauma lesions, are not presented.
The SEFFI (Superficial Enhanced Fluid Fat Injection) is a standardized technique and published that aims to harvest and re-inject autologous microfragmented adipose tissue with minimum manipulation. In this study, the harvesting and preparation procedure was performed by a disposable medical device SEFFILLER™ (Produced by SEFFILLINE srl Bologna Italy) compared with a traditional method. The advantages of the micrograft are the safety, reproducibility, and standardization of the procedure, the possibility of implanting fatty tissue at the surface level even with needles having a high content of stem cells.
It has been demonstrated by previous research that the use of this method leads to adipose tissue samples in which mature viable adipocytes are present, statistically more vital, and metabolically active with intact cell walls and visible nuclei, in addition to a well-represented stromal component between adipocytes, without signs of cell necrosis.
However, to explain the good clinical results achieved with this method, we asked ourselves if this activity is exclusively related to the trophic, anti-fibrotic, and revascularization properties of SVF/ADSCs or is also due to the presence of secreted bioactive molecules such as exosomes.
The mechanism of action of ADSCs in vitro and in vivo may be conflicting, and paracrine effects may be associated with both secreted vesicles and soluble factors, so the use of extracellular vesicles alone would limit the stimulatory and regenerative effects of ADSCs.
Exosomes have been found for many years to play an important role in cell-to-cell communication and immunological function, and in addition, they have shown many potential clinical applications, including stem cell maintenance and plasticity, biomarkers, and wound healing supplements that promote angiogenesis, we focused on exosomes presence in fat graft samples.
According to reports, human-induced pluripotent stem cells produce exosomes that can enhance skin fibroblast aging. The key to the clinical practice of medicine is the ability of exosomes to communicate intracellularly, leading to wound healing, immunomodulation, signaling and cell differentiation, and metabolic reprogramming.
The results are very interesting because, in addition to exosomes’ high presence, their miRNA content was also found, which indicates a large number of regenerative, anti-inflammatory, and anti-aging properties.
This evidence, related to the increased production of collagen, elastin, and hyaluronic acid in fibroblasts in vitro after the application of exosomes derived from fat samples, can even better explain the clearer clinical outcome of one method compared to the other.
The use of exosomes in medicine is receiving increasing attention and it is an area of growing interest in plastic surgery. Although starting from a clinical observation, the present work focused on determining the presence of exosomes in the adipose graft, as a determining factor in the success of the clinical result obtained.
There could potentially be a discrepancy between the mechanism of action of ADSCs in vitro and in vivo, and paracrine effects can be attributed to both secreted vesicles and soluble factors, so the possible use of extracellular vesicles alone would limit the stimulatory and regenerative effects of mesenchymal stem cells. Key to clinical practice is the ability of exosomes to communicate intracellularly, leading to wound healing, immunomodulation, cellular differentiation, signal transduction, and metabolic reprogramming. Of note, exosomes derived from human-induced pluripotent stem cells have also been reported to enhance the aging of skin fibroblasts.
The in vivo, treatment with microfragmented autologous adipose tissue has demonstrated that it is a minimally invasive procedure with a very low rate of complications and is an antiaging medical treatment that combines efficacy, safety, and simplicity.
The in vitro finding of a more marked presence of exosomes in the final product derived from the SEFFI technique with an increase in the production of hyaluronic acid, type I collagen, and elastin, compared with other adipose tissue sampling methods, allows us to argue that this method is preferable to obtain more reproducible and stable clinical results. However, further clinical studies are needed to establish the impact, benefits, and effectiveness of exosomes in aesthetic plastic surgery.
Author contributions
Alessandro Casadei and Barbara Zavan made substantial contributions to the conception and design of the study and draft of the article and the final approval of the version to be published. Alessandro Casadei, Alessandro Gennai and Bruno Bovani made substantial contributions in selecting patients and performing surgical procedures and data acquisition; Barbara Zavan performed data analysis and interpretation; Tommaso Pusceddu, Lucia Sileo, Pia Cavaleri, Martina Greco made substantial contributions to comprehensive analysis on sample (specific molecular markers – TEM – scrutiny of miRNA content – in vitro fibroblasts treatment.
- Neuber G. Fat trasplantation. Verl Dtsch Ges Chir. Long Vern. 1893; 22:66.
- Pu LL, Yoshimura K, Coleman SR. Future Perspectives of Fat Grafting. Clin Plast Surg. 2015 Jul;42(3):389-94, ix-x. doi: 10.1016/j.cps.2015.03.007. Epub 2015 Apr 18. PMID: 26116945.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002 Dec;13(12):4279-95. doi: 10.1091/mbc.e02-02-0105. PMID: 12475952; PMCID: PMC138633.
- Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemother. 2016 Jul;43(4):268-274. doi: 10.1159/000448180. Epub 2016 Jul 26. PMID: 27721702; PMCID: PMC5040903.
- Fraser JK, Schreiber R, Strem B, Zhu M, Alfonso Z, Wulur I, Hedrick MH. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med. 2006 Mar;3 Suppl 1:S33-7. doi: 10.1038/ncpcardio0444. PMID: 16501628.
- Huang JI, Beanes SR, Zhu M, Lorenz HP, Hedrick MH, Benhaim P. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002 Mar;109(3):1033-41; discussion 1042-3. doi: 10.1097/00006534-200203000-00037. PMID: 11884830.
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007 Nov;213(2):341-7. doi: 10.1002/jcp.21200. PMID: 17620285.
- Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, Vesey G, Herbert BR. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012 Aug 22;10:172. doi: 10.1186/1479-5876-10-172. PMID: 22913454; PMCID: PMC3479070.
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001 Jan;28(1):111-9. PMID: 11248861.
- Mizuno H. Adipose-derived stem cells for regenerative medicine in the field of plastic and reconstructive surgery. J Oral Biosci. 2013;55(3):132-136. https://doi.org/10.1016/j.job.2013.04.005
- Morcos MW, Al-Jallad H, Hamdy R. Comprehensive Review of Adipose Stem Cells and Their Implication in Distraction Osteogenesis and Bone Regeneration. Biomed Res Int. 2015;2015:842975. doi: 10.1155/2015/842975. Epub 2015 Sep 13. PMID: 26448947; PMCID: PMC4584039.
- Luck J, Weil BD, Lowdell M, Mosahebi A. Adipose-Derived Stem Cells for Regenerative Wound Healing Applications: Understanding the Clinical and Regulatory Environment. Aesthet Surg J. 2020 Jun 15;40(7):784-799. doi: 10.1093/asj/sjz214. PMID: 31406975.
- Casadei A, Epis R, Ferroni L, Tocco I, Gardin C, Bressan E, Sivolella S, Vindigni V, Pinton P, Mucci G, Zavan B. Adipose tissue regeneration: a state of the art. J Biomed Biotechnol. 2012;2012:462543. doi: 10.1155/2012/462543. Epub 2012 Oct 3. PMID: 23193362; PMCID: PMC3488420.
- Coleman SR, Katzel EB. Fat Grafting for Facial Filling and Regeneration. Clin Plast Surg. 2015 Jul;42(3):289-300, vii. doi: 10.1016/j.cps.2015.04.001. PMID: 26116934.
- Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007 Apr 15;119(5):1409-1422. doi: 10.1097/01.prs.0000256047.47909.71. PMID: 17415234.
- Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier JL, Braye F, Damour O. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009 Sep;124(3):765-774. doi: 10.1097/PRS.0b013e3181b17b8f. PMID: 19730294.
- Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010 Nov;63(11):1886-92. doi: 10.1016/j.bjps.2009.10.028. Epub 2009 Dec 7. PMID: 19969517.
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009 Oct;124(4):1087-1097. doi: 10.1097/PRS.0b013e3181b5a3f1. PMID: 19935292.
- Lin JY, Wang C, Pu LL. Can we standardize the techniques for fat grafting? Clin Plast Surg. 2015 Apr;42(2):199-208. doi: 10.1016/j.cps.2014.12.005. Epub 2015 Feb 20. PMID: 25827564.
- Trivisonno A, Di Rocco G, Cannistra C, Finocchi V, Torres Farr S, Monti M, Toietta G. Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J. 2014 May 1;34(4):601-13. doi: 10.1177/1090820X14528000. Epub 2014 Mar 31. PMID: 24687265.
- Rubina K, Kalinina N, Efimenko A, Lopatina T, Melikhova V, Tsokolaeva Z, Sysoeva V, Tkachuk V, Parfyonova Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009 Aug;15(8):2039-50. doi: 10.1089/ten.tea.2008.0359. PMID: 19368510.
- Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009 Jan;27(1):230-7. doi: 10.1634/stemcells.2008-0273. PMID: 18772313; PMCID: PMC2936459.
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005 Jul 1;332(2):370-9. doi: 10.1016/j.bbrc.2005.04.135. PMID: 15896706.
- Akita S, Akino K, Hirano A, Ohtsuru A, Yamashita S. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int. 2010 Dec 1;2010:532704. doi: 10.4061/2010/532704. PMID: 21151652; PMCID: PMC2995929.
- Gennai A, Baldessin M, Melfa F, Bovani B, Camporese A, Claysset B, Colli M, Diaspro A, Russo R, Strano P, Bollero D, Capparè G, Casadei A, Gallo G, Piccolo D, Salti G, Tesauro P. Guided Superficial Enhanced Fluid Fat Injection (SEFFI) Procedures for Facial Rejuvenation: An Italian Multicenter Retrospective Case Report. Clin Pract. 2023 Aug 8;13(4):924-943. doi: 10.3390/clinpract13040085. PMID: 37623266; PMCID: PMC10453478.
- Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E, Baldelli I, Fraternali-Orcioni G, Hartstein ME, Santi PL, Quarto R. Superficial Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging of the Face and Periocular Region. Aesthet Surg J. 2015 Jul;35(5):504-15. doi: 10.1093/asj/sjv001. Epub 2015 Apr 24. PMID: 25911629.
- Rossi M, Roda B, Zia S, Vigliotta I, Zannini C, Alviano F, Bonsi L, Zattoni A, Reschiglian P, Gennai A. Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthet Surg J. 2020 May 16;40(6):679-690. doi: 10.1093/asj/sjy142. PMID: 29905790.
- Gennai A, Bovani B, Colli M, et al: Comparison of Harvesting and Processing Technique for Adipose Tissue Graft: Evaluation of Cell Viability. Int J Regen Med. 2021; 4(2):2-5. https://doi.org/10.31487/j.RGM.2021.02.03
- H Rashed M, Bayraktar E, K Helal G, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017 Mar 2;18(3):538. doi: 10.3390/ijms18030538. PMID: 28257101; PMCID: PMC5372554.
- Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989 Oct;74(5):1844-51. PMID: 2790208.
- Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, Xu T, Zhang X, Lin C, Gao W, Guo Y, Lei B. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano. 2019 Sep 24;13(9):10279-10293. doi: 10.1021/acsnano.9b03656. Epub 2019 Sep 9. PMID: 31483606.
- Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE, Li S, Kao HK, Guo L. Exosomes Are Comparable to Source Adipose Stem Cells in Fat Graft Retention with Up-Regulating Early Inflammation and Angiogenesis. Plast Reconstr Surg. 2019 Nov;144(5):816e-827e. doi: 10.1097/PRS.0000000000006175. PMID: 31385891.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 Feb 7;367(6478):eaau6977. doi: 10.1126/science.aau6977. PMID: 32029601; PMCID: PMC7717626.
- Zhang H, Song P, Tang Y, Zhang XL, Zhao SH, Wei YJ, Hu SS. Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg. 2007 Nov;134(5):1234-40. doi: 10.1016/j.jtcvs.2007.07.019. PMID: 17976455.
- Hale SL, Dai W, Dow JS, Kloner RA. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes: a quantitative assessment. Life Sci. 2008 Sep 26;83(13-14):511-5. doi: 10.1016/j.lfs.2008.07.020. Epub 2008 Aug 9. PMID: 18755200; PMCID: PMC2582905.
- Ku YC, Omer Sulaiman H, Anderson SR, Abtahi AR. The Potential Role of Exosomes in Aesthetic Plastic Surgery: A Review of Current Literature. Plast Reconstr Surg Glob Open. 2023 Jun 12;11(6):e5051. doi: 10.1097/GOX.0000000000005051. PMID: 37313480; PMCID: PMC10259637.