More Information
Submitted: June 14, 2025 | Approved: July 23, 2025 | Published: July 24, 2025
How to cite this article: Jaramillo FJ, Coronel A, Zapata DM, Toro-Pedroza A, Tarapues EM, Useche E, et al. Outcomes of Standard-dose vs. Low-dose Total Body Irradiation for Allogeneic Stem Cell Transplantation in Adults with Acute Lymphoblastic Leukemia. J Stem Cell Ther Transplant. 2025; 9(2): 031-036. Available from:
https://dx.doi.org/10.29328/journal.jsctt.1001049
DOI: 10.29328/journal.jsctt.1001049
Copyright License: © 2025 Jaramillo FJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Total body irradiation; Allogeneic hematopoietic cell transplantation; Myeloablative conditioning; Hematologic malignancies; Leukemia
Outcomes of Standard-dose vs. Low-dose Total Body Irradiation for Allogeneic Stem Cell Transplantation in Adults with Acute Lymphoblastic Leukemia
Francisco J Jaramillo1*, Anabeli Coronel2, Diana M Zapata2, Alejandro Toro-Pedroza3, Eliana Manzi Tarapues4, Elena Useche4, Diana M Muñoz4 and Joaquín D Rosales1
1Department of Hematology, Bone Marrow Transplant Unit, Valle del Lili Foundation, Cali, Colombia
2Hematology and Clinical Oncology, ICESI University, Cali, Colombia
3Faculty of Health Sciences, ICESI University, Cali, Colombia
4Clinical Research Center (CIC), Valle del Lili Foundation, Cali, Colombia
*Address for Correspondence: Francisco J Jaramillo, Department of Hematology, Bone Marrow Transplant Unit, Hospital University Foundation Valle del Lili. Street number 98 No. 18-49 Cali 760032, Colombia, Email: [email protected]
Background: Conditioning regimens for allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) in adult Acute Lymphoblastic Leukemia (ALL) typically involve administering a ≥ 12 Gy dose of Total Body Irradiation (TBI), offering survival benefits but with potential acute and long-term complications. Within Myeloablative Conditioning (MAC) regimens, the optimal TBI dose in terms of outcomes and safety remains unknown, and the economic challenges of the standard approaches are pronounced in Low-middle Income Countries (LMIC). To address this gap, this study analyzes the outcomes of high-dose versus low-dose TBI in adult ALL patients undergoing allo-HSCT in Cali, Colombia.
Objectives: To compare the clinical outcomes of Myeloablative Conditioning (MAC) regimens based on a standard-dose TBI of ≥ 12 Gy (SD-TBI) versus regimens with low-dose TBI ≤ 4 Gy (LD-TBI) in adult patients with ALL and identical and haploidentical allo-HSCT.
Patients and methods: A retrospective cohort study was conducted with adult patients (≥18 years of age) with ALL between 2012 and 2021, who underwent allo-HSCT at a single center, in Cali-Colombia. The study population was divided into two MAC therapy groups: SD-TBI (≥ 12 Gy TBI) plus Flu and LD-TBI (≤ 4 Gy TBI) plus Bu and Flu. The primary outcome was Overall Survival (OS). Secondary outcomes included Disease-free Survival (DFS), Cumulative Incidence of Relapse (CIR), non-relapse mortality (NRM), Transplant-related Mortality (TRM), and acute and long-term transplant-associated complications. Outcomes were assessed at 12 months.
Results: A total of 100 patients were included, 39 received SD-TBI and 61 LD-TBI. Median age was 30 years, 97% of patients were classified as high risk, and only 49% were in first Complete Response (CR1). OS at 12 months was 59% and 47% in the SD-TBI and LD-TBI groups, respectively (p = 0.305). Relapse incidence at 12 months was 29% and 38.5% (p = 0.442). The incidence of grade II-IV acute graft versus host disease (aGVHD) was 24% in the SD-TBI vs. 46% in the LD-TBI group (p = 0.028). The relative risk (RR) for GVHD in the SD-TBI group was 0.52 (95% CI 0.27-0.98). The adjRR for donor type was 0.9 (95% CI 0.45-1.9) for haploidentical transplantation and RR 0.3 (95% CI 0.08 - 0.82) for HLA identical.
Conclusion: In ALL patients taken to allo-HSCT between 2012 and 2021, we found no significant differences in OS, DFS, TRM, or relapse incidence between SD-TBI and LD-TBI MAC regimens. The risk of aGVHD was lower in patients with SD-TBI, particularly those with HLA-identical donors. These results enhance Latin American representation in transplantation studies and have potential clinical implications. This study also serves as a base for future prospective studies and cost-effectiveness analyses needed for optimization of allo-HSCT conditioning regimens for ALL in LMIC.
Acute Lymphoblastic Leukemias (ALL) are a group of heterogeneous hematologic diseases representing 0.3% of all neoplasms [1]. In the management of high-risk ALL, allo-HSCT plays a critical role and TBI is an integral part of myeloablative conditioning (MAC) regimens before transplantation, with a recommended standard dose of 12 Gy [2]. Some advantages of TBI include the homogeneous management of sanctuary sites, such as the Central Nervous System (CNS), testis, and medullary niches. However, many acute and late complications have been reported, including pulmonary, ocular toxicity, and secondary neoplasms [2]. Currently, various modalities for TBI delivery exist, with differences among centers and countries in terms of prescribed doses, patient positioning and immobilization, organ shielding, and radiation therapy techniques [3]. Therefore, the optimal TBI dose for maximizing benefits while minimizing toxicity in MAC remains debated due to the extreme heterogeneity of the data [4].
MAC has also been reported as one of the major determinants of overall costs for allo-HSCT [5], mainly due to its higher complexity, prolonged hospital stay, and related complications [6]. In LMICs, challenges are intensified due to limited access to advanced medical technologies and facilities for TBI delivery and precise MAC regimens, leading to inconsistent treatment protocols and suboptimal outcomes. Thus, there is a critical need for conditioning regimens that are effective, safe, cost-efficient, and feasible to implement in LMICs.
This retrospective study aims to compare the clinical outcomes of two distinct Total Body Irradiation (TBI)-containing Myeloablative Conditioning (MAC) regimens in adult patients diagnosed with Acute Lymphoblastic Leukemia (ALL) undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT); specifically, the study evaluates the impact of a standard-dose TBI regimen (≥12 Gy) combined with fludarabine (SD-TBI) versus a low-dose TBI regimen (≤4 Gy) combined with busulfan and fludarabine (LD-TBI).
The primary objective is to determine whether there are significant differences in overall survival, relapse incidence, and treatment-related toxicity between both regimens.
A single-center retrospective cohort study was conducted, which included all adult patients (≥18 years) with ALL who underwent allo-HSCT from 2012 to 2021, at Fundación Valle del Lili, an academic hospital and high complexity center in Cali-Colombia. Patients who met the inclusion criteria were sampled from the total of patients managed in the hospital’s hematology clinic during the mentioned period. The study was approved by the biomedical research ethics committee of the institution (code 2018.1207). The data were obtained from electronic clinical records in the institutional software.
The choice of conditioning regimen was determined by clinical judgement of the treating physician based on several factors, including the patient’s age, disease status, comorbidities, previous treatment history, the toxicity profile of the regimen, the likelihood of achieving remission, and the resources available. Two groups were established: one group received SD-TBI ≥ 12 Gy plus fludarabine (120 mg/m2), and the second group LD-TBI-based regimens, which included < 4 cGy busulfan (3.2 mg/kg, 2 days) and fludarabine (30 mg/m2, 3 days). Per protocol, hematopoietic progenitor cell infusion was performed at a dose between 2-10 million hematopoietic progenitor cells per kilogram of recipient bodyweight.
GVHD prophylaxis included cyclosporine from day -1 to 120, then tapering. Mycophenolate mofetil 500 mg tid from day +14 and cyclophosphamide 60 mg/kg/d from day +3 and +4 post allo-HSCT.
The primary outcome analyzed was OS, and the secondary outcomes were DFS, NRM, TRM, and acute and long-term transplant-associated complications. All outcomes were assessed at 12 months. OS was defined as the period from transplantation to death from any cause, and DFS as the period from transplantation to disease relapse or death [7,8]. TRM refers to the death of a patient in the first 100 days from the procedure by any cause different from relapse of the disease.
Statistical analysis
Descriptive analysis was performed for all variables. Quantitative variables were summarized using median and interquartile range (IQR), while qualitative variables were presented with absolute frequencies and percentages. Statistically significant differences were evaluated using hypothesis tests, with a p value < 0.05 considered significant. For quantitative variables, the Mann-Whitney test was used, and for qualitative variables, the Chi-square test or Fisher’s exact test was employed when applicable. The Relative Risk (RR) association measure was calculated, and the Mantel-Haenszel test was used to adjust for potential confounding or effect-modifying variables. Overall Survival (OS) and cumulative incidence of acute (aGVHD) were calculated using the Kaplan-Meier method, with differences between groups assessed using the Log-rank test. For the analysis related to aGVHD, 97 patients with neutrophil and platelet engraftment were considered. Calculations related to chronic (cGVHD) included 80 patients who survived up to day 100. The statistical analysis was performed using Stata 14.0.
From 2012 to 2021, 100 adult patients (≥18 years) underwent allo-HSCT for adult ALL. The median age was 30 years (range 18-62 years). Eighty-three percent of patients had a diagnosis of ALL type B, 12% were BCR/ABL positive, and 12% had CNS involvement at diagnosis.
In all cases, hematopoietic progenitor cells were obtained from peripheral blood of related donors, 44% being HLA identical and 56% being haploidentical. At the time of transplantation, 49% were in first complete response, 37% were in second complete response or more, 11% had active disease, and in 3%, the status could not be established. Additional baseline characteristics are shown in Table 1.
| Table 1: Clinical characteristics stratified by conditioning regimen. | ||||
| Variable | Total, n = 100 | TBI (≥ 1200 cGy) N = 39 | TBI (≤ 400 cGy) N = 61 | p - value |
| Age | ||||
| Median (IQR) Range (Min - Max) |
30 (24-43) 18-62 |
34 (24-43) 18-58 |
30 (25-42) 18-62 |
0.576 |
| Sex | ||||
| Male n (%) | 58 (58) | 21 (54) | 37 (61) | 0.501 |
| CNS involvement, n (%) | 12 (12) | 4 (10.3) | 8 (13) | 0.872 |
| Philadelphia chromosome n (%) | 12 (12) | 3 (7.7) | 9 (14.7) | 0.289 |
| Risk stratification (%) | ||||
| Standard risk High risk |
3 (3) 97 (97) |
1 (2.6) 38 (97.4) |
2 (3.3) 59 (96.7) |
|
| Disease status at the time of transplantation (%) | ||||
| Not known 1 complete answer ≥2 complete responses Without establishing |
3 (3) 49 (49) 37 (37) 11 (11) |
2 (5) 22 (56) 10 (26) 5 (13) |
1 (1.7) 27 (44) 27 (44) 6 (9.8) |
0.250 |
| Immunophenotype, n (%) | ||||
| LLA-B LLA-T LLA-Biclonal SD |
83 (83) 7 (7) 1 (1) 9 (9) |
32 (82) 2 (5) 0 5 (13) |
51 (84) 5 (8.2) 1 (1.6) 4 (6.6) |
0.572 |
| Donor Age | ||||
| Median (IQR) Range (Min - Max) |
33 (25-45) 9-69 |
36 (24-46) 9-57 |
30 (25-42) 14-69 |
0.557 |
| Type of donor, n (%) | ||||
| Identical related (9/10 -10/10) Haploidentical related (5/10-8/10) |
44 (44) 56 (56) |
23 (59) 16 (41) |
21 (34) 40 (66) |
0.016 |
| Donor/recipient sex n (%) | ||||
| Male/female Female/female Male/male Female/male |
27 (27) 15 (15) 43 (43) 15 (15) |
8 (20) 10 (26) 17 (44) 4 (10) |
19 (31) 5 (8) 26 (43) 11 (18) |
0.079 |
| ABO incompatibility n (%) | ||||
| Compatible Major Minor |
75 (75) 14 (14) 11 (11) |
27 (69) 9 (23) 3 (8) |
48 (79) 5 (8) 8 (13) |
0.096 |
| CMV IgG n (%) | ||||
| D-/R- D+/R- D-/R+ D+/R+ |
0 1 (1) 13 (13) 86 (86) |
0 1 (2) 3 (8) 35 (90) |
0 0 10 (16) 51 (84) |
0.217 |
| IQR: Interquartile Range; CNS: Central Nervous System; ALL: Acute Lymphoid Leukemia; ND: No data; CMV: Cytomegalovirus; D: Donor; R: Recipient. | ||||
Thirty-nine patients received SD-TBI and 61 received LD-TBI-based regimens. All but three patients achieved hematopoietic reconstitution; these three patients died without engraftment. The median time to neutrophil engraftment was 16 days (interquartile range [IQR], 14 - 18) and to platelet engraftment was 16 days (IQR, 13 - 21) in the entire cohort, with no statistically significant differences between the groups.
Overall survival
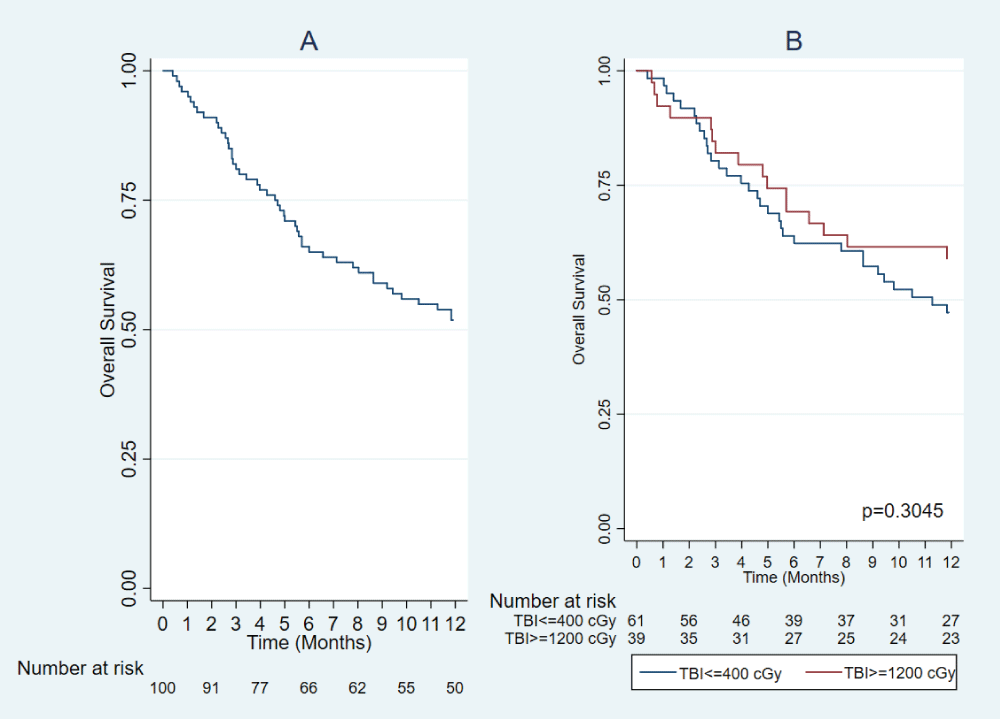
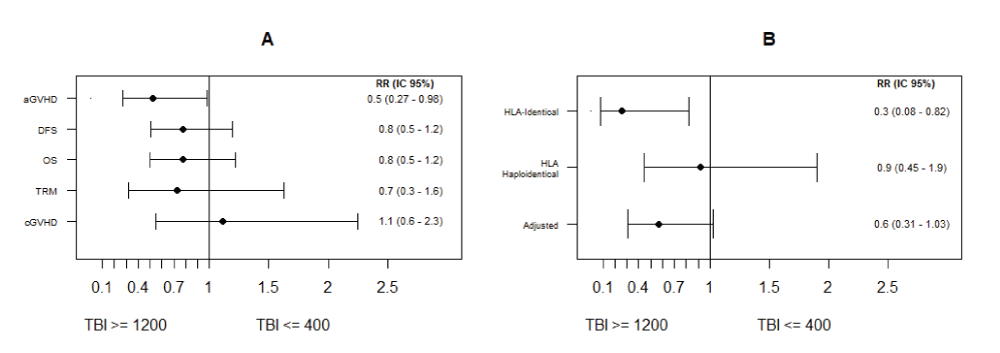
The 12-month OS of the entire cohort was 52% (95% CI: 42-61). For patients receiving SD-TBI, the 12-month OS was 59% (95% CI: 42-72) compared to 47% (95% CI: 34-59) in the group receiving LD-TBI, p = 0.305 (Figure 1). The relative risk for all-cause mortality comparing the use of SD-TBI vs. LD-TBI was 0.78 (95%CI: 0.5-1.22), p = 0.264 (Figure 2A).
Figure 1: Overall post-transplant survival (A). Overall survival according to the use of TBI ≥ 1200 cGy and TBI ≤ 400 cGy (B).
Figure 2: Relative risk and 95% confidence intervals for OS, DSF MRD, aGVHD, and cGVHD (A). Stratified analysis for aGVHD by donor type (B).
Secondary outcomes
There were no significant differences in relapse incidence, DFS, or NRM between the two groups. The 12-month CIR in the entire cohort was 35% (95%CI: 25-46), with 29% (95%CI:17-48) and 38.5% (95%CI: 26-54) of patients relapsing in the SD-TBI and LD-TBI groups, respectively (p = 0.4420). TRM in the whole cohort was 13%, with 10% in the SD-TBI group and 15% in the LD-TBI group, with no significant differences between the two groups (p = 0.31).
The overall 12-month DFS was 48.9% (CI95: 39-58), 56.4% in the SD-TBI (95%CI: 39-70), and 44% in the LD-TBI group (95%CI: 31-56) (p = 0.2678). The RR for DSF comparing the use of SD-TBI vs. LD-TBI was 0.78 (95%CI: 0.51-1.2), p = 0.236 (Figure 2A).
Toxicity and complications
The proportion of patients with GVHD II-IV was lower in the SD-TBI group (24% vs. 46%; RR 0.52, 95%CI: 0.27-0.97; p = 0.028). Table 2, Figure 2A. After adjusting for donor type, the adjusted RR is 0.57, 95% CI 0.31-1.03. The RR estimates in the stratified analysis for donor type were: Identical c and Haploidentical 0.92 (0.45-1.9). The Mantel-Haenzel homogeneity test resulted in a value of p = 0.061. Figure 2B.
| Table 2: Complications associated with transplantation. | ||||
| Variable | Total, n = 100 | TBI (≥1200 cGy) N = 39 | TBI (≤400 cGy) N = 61 | p - value |
| Acute GVHD (n = 97) n (%) Grade II-IV |
36 (37) | 9 (24) | 27 (46) | 0.028 |
| Type of acute GVHD, n (%) Cutaneous Gastrointestinal Hepatic |
18 (47) 20 (53) 9 (24) |
2 (22) 5 (56) 2 (22) |
16 (55) 15 (52) 7 (24) |
0.084 0.721 0.992 |
| Chronic GVHD (n = 80) n (%) Grade I Grade II-III |
24 (30) 11 17 |
10 (31) 6 4 |
14 (29) 5 13 |
0.842 |
| Hemorrhagic cystitis n (%) Grade II-IV Polyomavirus |
28 (28) 11 (39) |
9 (23) 1 (11) |
19 (31) 10 (53) |
0.381 0.036 |
| Cytomegalovirus, n (%) Viremia + Treatment CMV disease |
70 (70) 17 |
24 (61) 6 |
46 (75) 11 |
0.140 |
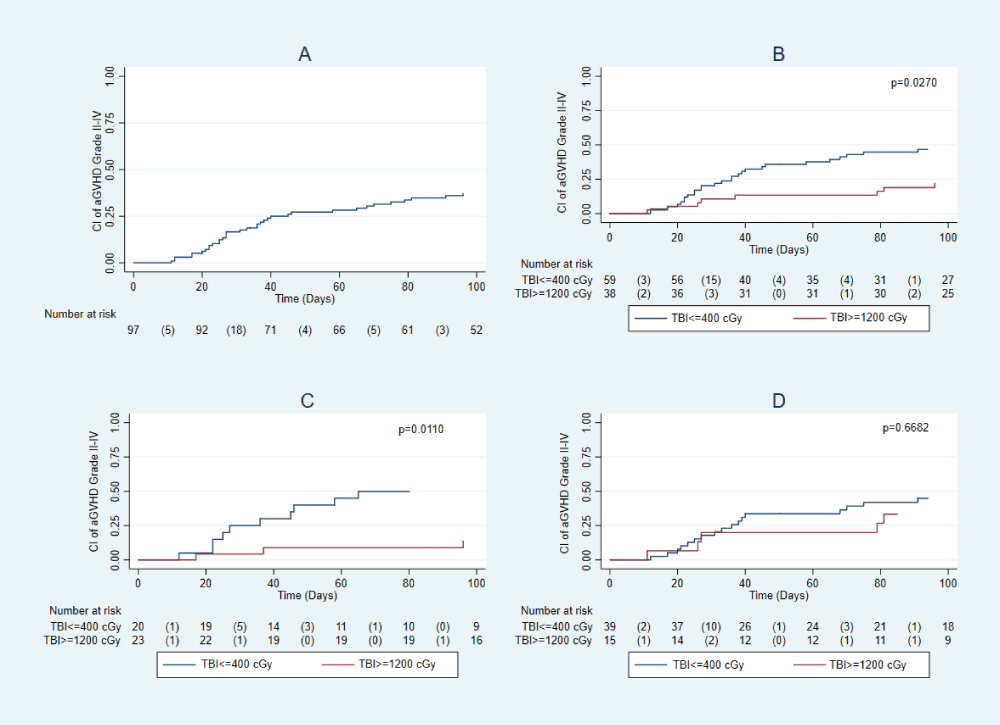
cGVHD presented in 33% of patients, with no significant difference between groups. Cumulative incidence according to TBI dose regimen, and HLA identical/haploidentical donors is shown in Figure 3. The incidence of grade II-IV hemorrhagic cystitis was 28% in the entire cohort, with 23% in the SD-TBI group and 31% in the LD-TBI group. This difference was not statistically significant (p = 0.381).
Figure 3: Cumulative incidence (AI) of aGVHD (A). Cumulative incidence of aGVHD according to the use of TBI ≥ 1200 and TBI ≤ 400 (B). Cumulative incidence of aGVHD according to the use of TBI ≥ 1200 and TBI ≤ 400 in HLA-identical recipients (C). Cumulative incidence of GVHD according to the use of TBI ≥ 1200 and TBI ≤ 400 in HLA Haploidentical recipients (D).
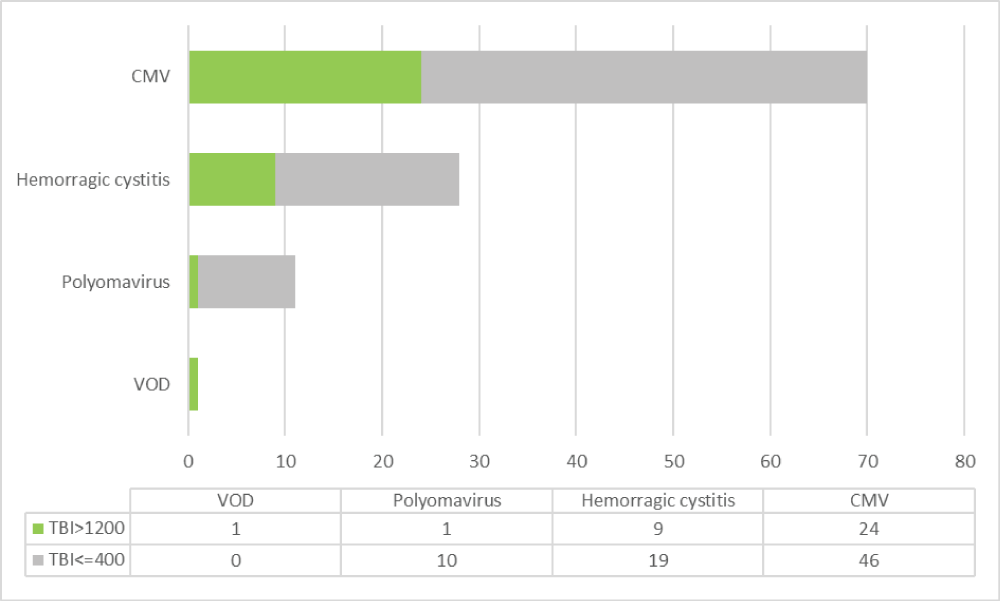
Cytomegalovirus viremia was detected in 70% of patients, 24% of whom required treatment. There were no significant differences between the two groups (p = 0.140) (Figure 4). There were 77 episodes of bacterial, viral (non-CMV), and fungal infections, corresponding to 53 patients during the first 100 days after transplantation. One patient in the SD-TBI group developed fatal hepatic veno-occlusive disease.
Figure 4: Complications during 12 months post-TAPH according to the use of TBI ≥ 1200 and TBI ≤ 400. CMV: Cytomegalovirus Viremia + treatment, VOD: Veno-occlusive Disease.
This retrospective study conducted at a single center analyzed 100 adult patients with ALL who underwent allo-HSCT, using two different TBI-based MAC regimens. No significant differences were found in OS (p = 0.305), DFS (p = 0.23), or cumulative incidence of relapse (p = 0.32) between the SD-TBI and LD-TBI groups. The risk of aGVHD was lower in patients with SD-TBI, specifically in patients with HLA-identical donors (RR 0.26, 95% CI 0.08-0.82). This suggests that SD-TBI MAC regimens have comparable outcomes to LD-TBI and are associated with a lower incidence of aGVHD.
The EBMT and EWALL recommend MAC TBI-based regimens for ALL patients, a stance also supported by the American Society for Transplantation and Cellular Therapy [9,10]. These recommendations are based on large retrospective studies showing that TBI-based regimens significantly improve PFS and OS compared to chemotherapy-only conditioning [11–17]. The EBMT reported significantly reduced NRM at 2 years and improved Leukemia-free survival (LFS) with TBI-based conditioning in the setting of haploidentical HSCT with post-transplant cyclophosphamide, although the overall survival benefit was not significant [18]. Other studies have found that fludarabine-TBI reduced NRM compared to thiotepa, busulfan, and fludarabine (TBF), but increased relapse risk without affecting OS or GVHD. This suggests that fludarabine could be a reasonable alternative to cyclophosphamide and/or etoposide in TBI-based MAC [19,20]. However, some controversy exists regarding the survival advantage of TBI-based conditioning regimens [3]. A randomized study of ALL patients in CR1 receiving either Bu-Cy or TBI-Cy found Bu-Cy to be noninferior, with similar rates of relapse at 2 years and NRM. There were no significant differences in toxicity, GVHD, or late effects between the groups [21]. Thus, multiple conditioning schemes are used today in clinical practice, and the ideal regimen before allo-HCST in ALL remains controversial. Our results show that high-dose Flu-TBI 12 Gy does not significantly differ in outcomes and relapse incidence compared to lower intensity TBI (4 Gy) with Bu-Flu myeloablative chemotherapy regimens.
Although TBI has proven advantageous, it has been associated with both early and late adverse effects [22]. There are various modalities for delivering TBI, with significant differences regarding prescribed doses, patient positioning and immobilization, organ shielding, and radiation therapy techniques [3]. Consequently, the optimal TBI dose for achieving maximum benefits while minimizing toxicity in MAC remains a topic of debate, owing to the extreme heterogeneity of the available data [4]. In this study, one fatal case of hepatic veno-occlusive disease due to acute toxicity occurred in the SD-TBI group. While no additional significant TBI-related toxicities were reported in the study, the occurrence of such severe liver toxicity highlights the importance of optimizing conditioning regimens of patients undergoing allo-HSCT. Interestingly, we found statistically significant evidence of reduced relative risk for aGVHD grade II-IV with TBI ≥12 Gy versus TBI ≤ 4 Gy. When performing the analysis adjusted by type of donor (identical and haploidentical), it is statistically significant only in patients taken to HLA identical transplantation with SD-TBI. This study suggests HLA identical allo-HSCT recipients have a lower risk of aGVHD when receiving TBI-based regimens ≥12 Gy, although these findings might be influenced by the retrospective nature of the study and the sample size.
Globally, the number of allogeneic transplants is increasing, particularly in treating conditions like ALL, where they offer a curative possibility. However, the cost of these procedures, especially in countries with emerging economies, poses a significant challenge for public health systems [23]. MAC has also been reported as one of the major determinants of overall costs for allo-HSCT. Mainly due to its higher complexity, prolonged hospital stay, and related complications [24]. At our institution, costs are individualized by drug and radiation therapy. Based on unpublished intrainstitutional data, we have observed that costs are reduced when conditioning TBI >12 Gy is used. As high-cost treatments become accessible to more patients, it is crucial not only to evaluate clinical outcomes but also to manage financial costs effectively. Therefore, in LMIC, future cost-effectiveness studies are essential to determine if certain conditioning regimens offer financial advantages over others.
In the treated population, which consisted entirely of Latin American Hispanics, 98% of the patients were classified as high-risk based on available clinical and paraclinical criteria. Both identical and haploidentical donors were included for transplantation, with 49% of the patients being in CR1. As a result, this study encompassed patients with very high-risk profiles and poor prognostic factors, which explains the lower 12-month survival outcomes in our cohort compared to other centers.
Limitations of this study are primarily related to the retrospective nature of the data. There was substantial heterogeneity in the patient population diagnosis. Access to molecular profiling is also limited in our context. In addition, during the 9 years during which the data were collected, management likely changed. This may introduce a potential bias. Because this was a retrospective study, there also may have been differences in charting and reporting of various toxicities.
In ALL patients taken to allo-HSCT between 2012 and 2021, we found no significant differences in OS, DFS, TRM or relapse incidence between SD-TBI and LD-TBI MAC regimens. The risk of aGVHD was lower in patients with SD-TBI, particularly those with HLA-identical donors. These results enhance Latin American representation in transplantation studies and have potential clinical implications. This study also serves as a base for future prospective studies and cost-effectiveness analyses needed for optimization of allo-HSCT conditioning regimens for ALL in LMIC.
We thank Dr. Jaime Sanz Caballero, PhD, for his valuable advice, correction, and attention in relation to the Bone Marrow Transplant service of the Fundación Valle del Lili. His dedication and knowledge have been fundamental for the development of this work. We also thank Nurse Gloria Piedad Guerrero Fajardo of the Bone Marrow Transplant Service of the Fundación Valle del Lili, and Yensi Julieth Valencia of the Clinical Research Center for their support. Finally, we thank Dr. Juan P. Díaz-Solórzano for providing language editing and correction to improve the final version of the manuscript.
Declarations
Ethical approval: This study was reviewed and approved by the Institutional Review Board (IRB) of Fundación Valle del Lili. All procedures involving human participants were conducted by the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Data availability: The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical considerations: The authors declare that this study is not considered hazardous research according to international and national standards. Data collection was obtained from medical records.
- Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet Lond Engl. 2020;395(10230):1146–62. Available from: https://doi.org/10.1016/s0140-6736(19)33018-1
- Potdar RR, Gupta S, Giebel S, Savani BN, Varadi G, Nagler A, et al. Current Status and Perspectives of Irradiation-Based Conditioning Regimens for Patients with Acute Leukemia Undergoing Hematopoietic Stem Cell Transplantation. Clin Hematol Int. 2019;1(1):19–27. Available from: https://doi.org/10.2991/chi.d.190218.002
- Dogliotti I, Levis M, Martin A, Bartoncini S, Felicetti F, Cavallin C, et al. Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation. Cancers. 2024;16(5):865. Available from: https://doi.org/10.3390/cancers16050865
- Giebel S, Miszczyk L, Slosarek K, Moukhtari L, Ciceri F, Esteve J, et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120(17):2760–5. Available from: https://doi.org/10.1002/cncr.28768
- Jaime-Pérez JC, Heredia-Salazar AC, Cantú-Rodríguez OG, Gutiérrez-Aguirre H, Villarreal-Villarreal CD, Mancías-Guerra C, et al. Cost structure and clinical outcome of a stem cell transplantation program in a developing country: the experience in northeast Mexico. Oncologist. 2015;20(4):386–92. Available from: https://doi.org/10.1634/theoncologist.2014-0218
- Broder MS, Quock TP, Chang E, Reddy SR, Agarwal-Hashmi R, Arai S, et al. The Cost of Hematopoietic Stem-Cell Transplantation in the United States. Am Health Drug Benefits. 2017;10(7):366–74. Available from: https://pubmed.ncbi.nlm.nih.gov/29263771/
- Hahn T, Sucheston-Campbell LE, Preus L, Zhu X, Hansen JA, Martin PJ, et al. Establishment of Definitions and Review Process for Consistent Adjudication of Cause-specific Mortality after Allogeneic Unrelated-donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(9):1679–86. Available from: https://doi.org/10.1016/j.bbmt.2015.05.019
- Iacobelli S; On behalf of the EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48(S1):S1–37. Available from: https://doi.org/10.1038/bmt.2012.282
- DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic Cell Transplantation in the Treatment of Adult Acute Lymphoblastic Leukemia: Updated 2019 Evidence-Based Review from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25(11):2113–23. Available from: https://doi.org/10.1016/j.bbmt.2019.08.014
- Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019;54(6):798–809. Available from: https://doi.org/10.1038/s41409-018-0373-4
- Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543–8. Available from: https://doi.org/10.1038/sj.bmt.1704198
- Davies SM, Ramsay NK, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18(2):340–7. Available from: https://doi.org/10.1200/jco.2000.18.2.340
- Granados E, de La Cámara R, Madero L, Díaz MA, Martín-Regueira P, Steegmann JL, et al. Hematopoietic cell transplantation in acute lymphoblastic leukemia: better long term event-free survival with conditioning regimens containing total body irradiation. Haematologica. 2000;85(10):1060–7. Available from: https://pubmed.ncbi.nlm.nih.gov/11025598/
- Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22(14):2816–25. Available from: https://doi.org/10.1200/jco.2004.07.130
- Marks DI, Forman SJ, Blume KG, Pérez WS, Weisdorf DJ, Keating A, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438–53. Available from: https://doi.org/10.1016/j.bbmt.2005.12.029
- Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102(1):139–49. Available from: https://doi.org/10.3324/haematol.2016.145631
- Cahu X, Labopin M, Giebel S, Aljurf M, Kyrcz-Krzemien S, Socié G, et al. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2016;51(3):351–7. Available from: https://doi.org/10.1038/bmt.2015.278
- Dholaria B, Labopin M, Angelucci E, Tischer J, Arat M, Ciceri F, et al. Outcomes of Total Body Irradiation- Versus Chemotherapy-Based Myeloablative Conditioning Regimen in Haploidentical Hematopoietic Cell Transplantation with Post-Transplant Cyclophosphamide for Acute Lymphoblastic Leukemia: ALWP of the EBMT Study. Blood. 2019;134:320. Available from: https://doi.org/10.1182/blood-2019-122258
- Swoboda R, Labopin M, Giebel S, Angelucci E, Arat M, Aljurf M, et al. Total body irradiation plus fludarabine versus thiotepa, busulfan plus fludarabine as a myeloablative conditioning for adults with acute lymphoblastic leukemia treated with haploidentical hematopoietic cell transplantation. A study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022;57(3):399–406. Available from: https://doi.org/10.1038/s41409-021-01550-0
- Solomon SR, Solh M, Zhang X, Morris LE, Holland HK, Bashey A. Fludarabine and Total-Body Irradiation Conditioning before Ablative Haploidentical Transplantation: Long-Term Safety and Efficacy. Biol Blood Marrow Transplant. 2019;25(11):2211–6. Available from: https://doi.org/10.1016/j.bbmt.2019.06.017
- Zhang H, Fan Z, Huang F, Han L, Xu Y, Xu N, et al. Busulfan Plus Cyclophosphamide Versus Total Body Irradiation Plus Cyclophosphamide for Adults Acute B Lymphoblastic Leukemia: An Open-Label, Multicenter, Phase III Trial. J Clin Oncol. 2023;41(2):343–53. Available from: https://doi.org/10.1200/jco.22.00767
- Pearlman R, Hanna R, Burmeister J, Abrams J, Dominello M. Adverse Effects of Total Body Irradiation: A Two-Decade, Single Institution Analysis. Adv Radiat Oncol [Internet]. 2021;6(4):100723. Available from: https://doi.org/10.1016/j.adro.2021.100723
- Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120(8):1545–51. Available from: https://doi.org/10.1182/blood-2012-05-426783
- Pandit S, Sapkota S, Adhikari A, Karki P, Shrestha R, Jha DS, et al. Breaking barriers: supporting hematopoietic stem cell transplant program through collaborative radiation therapy service from a physically distant center. J Egypt Natl Cancer Inst. 2024;36(1):17. Available from: https://jenci.springeropen.com/articles/10.1186/s43046-024-00221-7