Research Article
Intraepidermal Injections of Autologous Epidermal Cell Suspension: A new promising approach to Dermatological Disorders. Preliminary Study
Elisa Borsani1,2, Luigi Fabrizio Rodella1,2, Elisabetta Sorbellini3, Rita Rezzani1,2, Daniela Pinto4, Barbara Marzani4, Giovanna Tabellini4, Mariangela Rucco3 and Fabio Rinaldi3*
1Department of Clinical and Experimental Sciences, Division of Anatomy and Physiopathology, University of Brescia, Viale Europa 11, 25123 Brescia, Italy
2Interdipartimental University Center of Research “Adaption and Regeneration of Tissues and Organs - (ARTO)”, University of Brescia, Viale Europa 11, 25123 Brescia, Italy
3International Hair Research Foundation (IHRF), Milan, Italy
4Department of Molecular and Translational Medicine, Division of Experimental Oncology and Immunology, University of Brescia, Viale Europa 11, 25123 Brescia, Italy
*Address for Correspondence: Dr. Fabio Rinaldi, International Hair Research Foundation (IHRF), Milan, Italy, Tel: 335-1488730; Email: [email protected]
Dates: Submitted: 24 November 2017; Approved: 06 December 2017; Published: 07 December 2017
How to cite this article: Borsani E, Rodella LF, Sorbellini E, Rezzani R, Pinto D, et al. Intraepidermal Injections of Autologous Epidermal Cell Suspension: A new promising approach to Dermatological Disorders. Preliminary Study. J Stem Cell Ther Transplant. 2017; 1: 066-070. DOI: 10.29328/journal.jsctt.1001007
Copyright License: © 2017 Borsani E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: ESCs: Epidermal Cells of the Interfollicular Epidermis (ESCs); PRP: Platelet Rich Plasma (PRP); SCs: Stem Cells
Abstract
Regenerative medicine is a modern approach of dermatological treatment, using Epidermal Cells of the interfollicular epidermis (ESCs) for their effect in skin regeneration in chronic ulcers and burns, melanoma, vitiligo, junctional epidermolysis bullosa. Intraepidermal injections of autologous epidermal cell suspension can be a new and very promising treatment for many other cutaneous disorders as non-scarring alopecia (Alopecia Areata, Androgenic Alopecia) or scarring alopecia (Lichern Plano Pilaris alopecia, Discoid Lupus Erithematosus alopecia), anti-aging therapies. The intraepidermal injection of an autologous epidermal cell suspension is a simple, fast and safe surgical procedure: a small, thin portion of the epidermis of the patient undergoes a treatment where a suspension with all the cells collected from the epidermis and cultured for 7 days is injected into the skin. Our preliminary study shows that a suspension contains a significant number of viable cells that survive at day 7 in culture.
Our research is ongoing and is focusing on the typing of the different cells in the suspension and evaluation of the presence and the nature of stem cells.
Introduction
Regenerative medicine is a modern approach of dermatological treatment, using Epidermal Cells of the interfollicular epidermis (ESCs) to help skin regeneration after severe damages, such as chronic ulcers and burns [1]. Some clinical trials have already investigated the use of ESCs to treat other skin diseases such as melanomas [2-5], Vitiligo [6-10] and Junctional Epidermolysis Bullosa [11].
The intraepidermal injection of an autologous epidermal cell suspension may be a new and extremely promising treatment for several other skin disorders, such as non-scarring alopecia (Alopecia Areata, Androgenic Alopecia), scarring alopecia (Lichen Plano Pilaris Alopecia, Discoid Lupus Erythematosus Alopecia), and anti-aging therapies. Our experience in autologous preparation like platelet rich plasma (PRP) in Alopecia Areata [12], a common auto-immune condition which causes hair loss induced by inflammation and presents limited therapeutic options, has encouraged us in the use of autologous derived material for helping skin regeneration and we started to investigate the effectiveness of epidermal cells against certain specific skin disorders.
Skin is a complex structure with two different layers, the epidermis and the dermis, made up of different cell lineages, which ensure the maintenance of the normal skin homeostasis (tissue repair, barrier function). In the basal layer of the epidermis, the cell population is heterogeneous as this is made up of several cell types such as keratinocytes, melanocytes, Langerhans cells, Merkell cells [13]. In the follicular epidermis, multiple populations of stem cells (SCs) are found in different locations, with niches of SCs in the basal layer of the epidermis, around the sebaceous gland and in the bulge niche of hair follicles [14,15].
The intraepidermal injection of an autologous epidermal cell suspension is a simple, fast and safe surgical procedure: a small, thin portion of the epidermis of the patient undergoes a treatment where a suspension with all the cells collected from the epidermis is injected into the skin. The first step, however, is to select the cells to be injected. So, the goal of this preliminary study was to investigate the content of an autologous suspension of epidermal cells and their viability after 7 days of culture and to find a simple and fast link between clinical application and in vitro manipulation of cells. Most interesting, this goal was achieved without using coated flask, as reported by other authors [16].
Results
Cell morphology
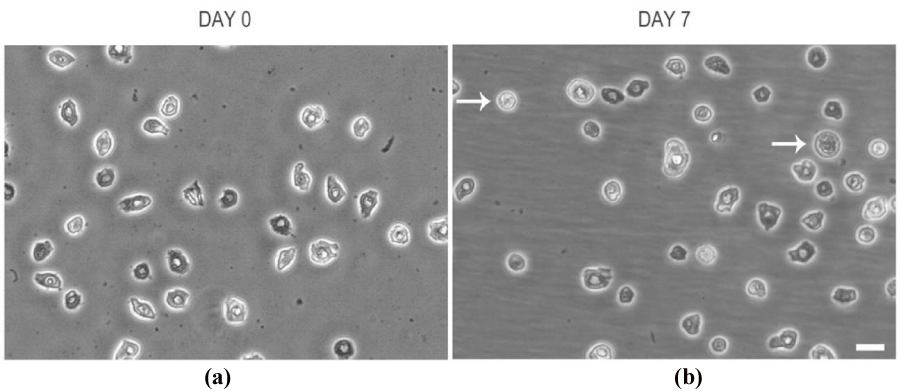
To investigate how cell morphology of epidermal cells in the suspension change during culturing we observed cell at 0 and 7 day of incubation by means of an inverted microscope. At day 0, the cells showed a heterogeneous appearance with regard to shape and size and their nucleus was almost detectable (Figure 1a). At day 7, the cells maintained a heterogeneous appearance with regard to shape and size, even though more spheroidal elements were observed. Cells were generally in suspension, only some microspots of fibroblastoid-shaped cells were well attached to the flask surface (Figure 1b).
Figure 1: Epidermal cells at a) day 0 and b) day 7 of culture. White arrows indicate spheroidal cell elements. Scale Bar=20 um.
Cytofluorimetric analysis for cell viability
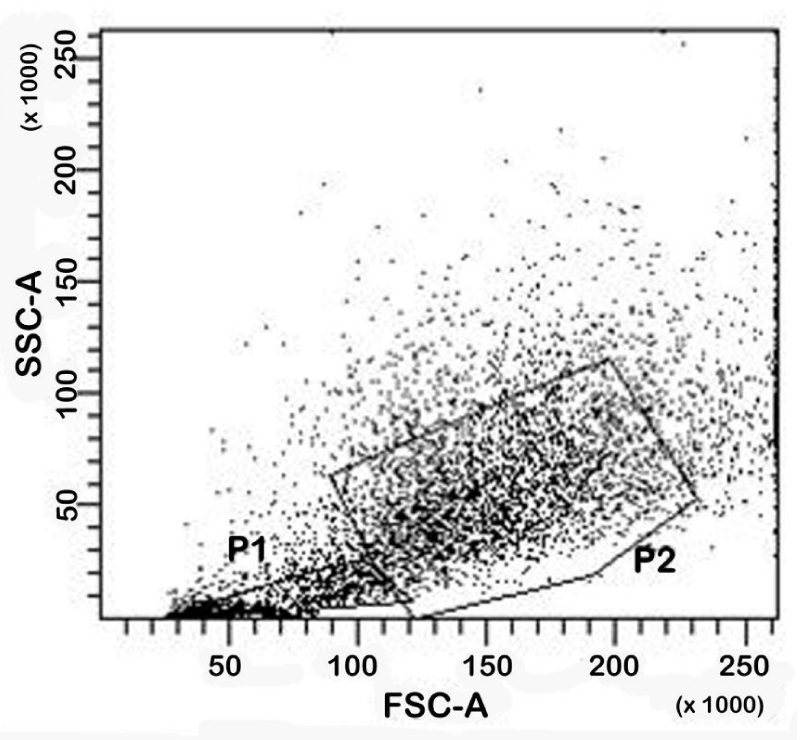
Cell viability was investigated by means of cytofluorimetric analysis. Representative dot-plot was reported in figure 2. The number of isolated cells at day 0 is shown in table 1. At this time point the percentage of viable cells was 59.40% (SD±6.07%), while the percentage of non-viable cells was 16.48% (SD±5.18%). The remaining cells formed clusters. At day 7 (data not shown), the percentage of viable cells was 63.55% (SD±5.41%), while the percentage of non-viable cells was 15.50% (SD±5.68%). The remaining cells formed clusters. These results show how the absolute percentage of viable and non-viable cells is maintained after 7 day of culture.
Figure 2: Cytofluorimetric analysis. A representative dot-plot of cell morphology at day 0. Gating cells in P1 are debris and death cells, gating cells in P2 are viable cells.
| Table 1: Number of isolated epidermal cells from 10 donors at day 0. | ||
| Subject # | Donor surface area (cm2) | # epidermal cells (103) |
| 1 | 5.9 | 325 |
| 2 | 6.3 | 475 |
| 3 | 5.7 | 720 |
| 4 | 6.2 | 483 |
| 5 | 4.6 | 700 |
| 6 | 5.3 | 580 |
| 7 | 6.1 | 650 |
| 8 | 5.7 | 734 |
| 9 | 6.2 | 580 |
| 10 | 4.7 | 315 |
| average | 5.7 | 556.63 |
Discussion
Autologous epidermal cell suspension injected into the skin may be a new approach to treat several skin disorders. Regenerative medicine could be applied in several medical fields, and dermatology may use this therapy to successfully treat specific disorders. This preliminary study shows that an autologous epidermal cells suspension contains a significant number of viable cells that survive at day 7 in culture.
Our research is currently continuing and it is focusing on the typing of the different cells in the suspension and evaluating the presence and the nature of stem cells. If biological studies will also confirm these data, it would be reasonable to think that the surgical procedure with an autologous epidermal cell suspension may offer several interesting opportunities for the near future. In summary, this procedure is simple and fast, lasting 60-90 minutes; there is a very limited risk of side effects (scars in the donor area, pain in the treated area due to the injections); it is a safe, not presenting risk of oncogenic disorders and/or immunologic reaction; is not expensive; is an outpatient treatment. However, some further studies are still needed to validate our findings in a larger cohort of skin samples and focus on the typing of the cells in the suspension. For the same reason, the actual effectiveness of this procedure needs to be validated by some controlled and randomized clinical trials.
Material and Methods
Collection of skin grafts
A graft of skin was collected from 10 healthy volunteers who agreed to undergo the study. All subjects provided their written informed consent before participating in the study, and the study was performed according to the Declaration of Helsinki. About six-ten square centimeters of 0.76 mm thick skin (Figure 3) were collected from the buttock of each subject under local epidermal anesthesia (lidocaine cl 20 mg/ml, 2 ml per area) through a Zimmer Biomet Electric Dermatome (Zimmer Biomet, Warsaw, Stati Uniti). The buttock area was chosen to strongly reduce the formation of scars and cause a milder cosmetic discomfort. Each sample was immediately immersed in a 70% ethanol solution for 30s to reduce contamination and washed with HBSS twice.
The epidermal grafts were then incubated in a Trypsin solution (Trypsin 0.5g/ EDTA 0.2 solution, Sigma Aldrich, Saint Louis, USA) for 45 minutes at 37°C (Plasmatherm Barkley, Leopoldshoehe, Germany). After incubation, the Trypsin solution was discarded and the tissues were washed with HBSS. The epidermis (thin yellow layer) was treated with a scalpel blade to separate the cells. The supernatant was suctioned through a sterile syringe and then cultured in Dulbecco′s modified Eagle′s medium (DMEM; Sigma Aldrich, Saint Louis, USA) supplemented with 10% (v/v) heat-inactivated foetal bovine serum (EuroClone, Devon, UK) and 1% penicillin/streptomycin solution (Sigma Aldrich, Saint Louis, USA) at 37°C in a humidified atmosphere of 95% air and 5% CO2. The cells were seeding in a 25 cm2 cell culture flask at a density of about 20000 cells/cm2. Cell morphology was evaluated before the seeding and after a 7 day culture by using an inverted Olympus microscope (Olympus Italia S.R.L., Milan, Italy). The protocols considered previously reported works [16-20].
Cytofluorimetric analysis for cell viability
Cells were centrifuged at 1000 rpm for 5 minutes. The supernatant was then removed. The pellet was re-suspended in 3 ml 4% paraformaldehyde in phosphate buffer 0.1 M pH, 7.4 for fixation. After 20 minutes at +4°C, the cells were centrifuged at 1000 rpm for 5 minutes and the pellet was re-suspended in 500 μl PBS. Finally, the cell samples were analysed with FACS (BD FACSCanto™ BD Bioscience, San Jose, CA) and the data were analysed by the BD FACSDiva™ software version 8.8.7 (BD Bioscience, San Jose, CA). An analysis was performed at day 0 and day 7 after the culture. The viable cells were identified using morphological parameters by choosing a range of cell size combined with morphological cell complexity.
References
- Jackson CJ, Tønseth KA, Utheim TP. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res Ther. 2017; 8: 155. Ref: https://goo.gl/x7nfCo
- University of Chicago. Chemotherapy plus peripheral stem cell transplantation in treating patients with melanoma or metastatic kidney cancer. ClinicalTrials.gov. 2014; NCT00004135.
- Hoerter JD, Bradley P, Casillas A, Chambers D, Weiswasser B, et al. Does melanoma begin in a melanocyte stem cell? J Skin Cancer. 2012. Ref: https://goo.gl/hxe7af
- Ann & Robert H Lurie Children’s Hospital of Chicago. Immunoablative mini transplant (hematopoietic peripheral blood stem cell transplant [HPBSC]). ClinicalTrials.gov. 2014; NCT00179764. Ref: https://goo.gl/xzTavg
- Fred Hutchinson Cancer Research Center. Combination chemotherapy, total-body irradiation, peripheral stem cell transplantation, and lymphocyte infusion in treating patients with Stage IV melanoma. ClinicalTrials.gov 2011; NCT00006233. Ref: https://goo.gl/RAzGH2
- Khodadadi L, Shafieyan S, Sotoudeh M, Dizaj AV, Shahverdi A, et al. Intraepidermal injection of dissociated epidermal cell suspension improves vitiligo. Arch Dermatol Res. 2010; 302: 593-599. Ref: https://goo.gl/PCCV95
- Van Geel N, Ongenae K, Naeyaert JM. Surgical techniques for vitiligo: a review. Dermatology. 2001; 202: 162-166. Ref: https://goo.gl/QJCLm9
- Lee DY, Lee JH. Epidermal grafting for vitiligo: a comparison of cultured and noncultured grafts. Clin Exp Dermatol. 2010; 35: 325-326. Ref: https://goo.gl/WMpBw6
- Guerra L, Capurro S, Melchi F, Primavera G, Bondanza S, et al. Treatment of "stable" vitiligo by Timedsurgery and transplantation of cultured epidermal autografts. Arch Dermatol. 2000; 136: 1380-1389. Ref: https://goo.gl/BZqtQk
- Matsuzaki K, Kumagai N. Treatment of vitiligo with autologous cultured keratinocytes in 27 cases. Eur J Plast Surg. 2013; 36: 651-656. Ref: https://goo.gl/SWQegA
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006; 12: 1397-1402. Ref: https://goo.gl/3rabfK
- Trink A, Sorbellini E, Bezzola P, Rodella L, Rezzani R, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013; 169: 690-694. Ref: https://goo.gl/8uhusp
- Allen TD, Potten CS. Fine-structural identification and organization of the epidermal proliferative unit. J Cell Sci. 1974; 15: 291-319. Ref: https://goo.gl/G1e472
- Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. The EMBO Journal. 2001; 20: 1215-1222. Ref: https://goo.gl/t4wVRJ
- Purba TS, Haslam IS, Poblet E, Jiménez F, Gandarillas A, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioassays. 2014; 36: 513-525. Ref: https://goo.gl/4Vte5v
- Gottipamula S, Saraswat SK, Sridhar KN. Comparative study of isolation, expansion and characterization of epithelial cells. Cytotherapy. 2017; 19: 263-271. Ref: https://goo.gl/CYY5kc
- De Kock J, Rodrigues RM, Buyl K, Vanhaecke T, Rogiers V. Human Skin-Derived Precursor Cells: Isolation, Expansion, and Hepatic Differentiation. Methods Mol Biol. 2015; 1250: 113-122. Ref: https://goo.gl/9qhxvg
- Fujimori Y, Izumi K, Feinberg SE, Marcelo CL. Isolation of small-sized human epidermal progenitor/stem cells by Gravity Assisted Cell Sorting (GACS). J Dermatol Sci. 2009; 56: 181-187. Ref: https://goo.gl/cAawnP
- Kaviani M, Geramizadeh B, Rahsaz M, Marzban S. Considerations in the improvement of human epidermal keratinocyte culture in vitro. Exp Clin Transplant. 2015; 13: 366-370. Ref: https://goo.gl/BY7Gsu
- Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998; 95: 3902-3907. Ref: https://goo.gl/A6EEhY